Abstract
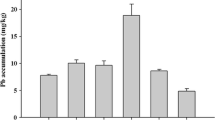
The present research was carried out to investigate the performance of oxalic acid (OA) through Lemna minor L. (duckweed) in the phytoextraction of lead (Pb) from metal contaminated water. Zero, 100 μM, 250 μM, and 500 μM Pb concentration and combinations with 2.5 ml of OA were provided to the plants in the form of solution after defining intervals. Continuous aeration was provided to the plants and kept a pH level at 6.5. Results from this research depicted that increasing concentration level of Pb inhibited the overall plant growth, biomass, frond area, chlorophyll, and antioxidant enzyme activities like peroxidase (POD), superoxide-dismutase (SOD), catalases (CAT), and ascorbate-peroxidase (APX). Moreover, Pb stress enhances the concentration, hydrogen peroxide, malondialdehyde, and electrolyte leakage substances in plants. Furthermore, the addition of OA alleviated the Pb-induced toxicity in the plants, increasing the Pb accumulation and its endorsement in the L. minor. The OA addition increased the Pb accumulation in plants at 0, 100, 250 and 500 μM. At higher concentration, Pb showed harmful effect as related to the other low doses. Under the application of OA, Pb higher accumulation and concentration in L. minor were measured, which showed that it could be the most suitable plant for the phytoextraction of lead-contaminated soil and wastewater.
Graphical abstract







Similar content being viewed by others
Data availability
This study is a master of philosophy lab scale research work, and the journal has full rights to publish this research. The data will be provided on the demand of the journal.
References
Aebi H (1984) Catalase in vitro. In: Methods in enzymology, vol 105. AP, pp 121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. ESPR. 22:11679–11689
Aken BV, Doty SL (2009) Transgenic plants and associated bacteria for phytoremediation of chlorinated compounds. Biotechnol Genet Eng Rev 26(1):43–64
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Zhou WJ (2013) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32(3):604–614
Ali S, Zeng F, Cai S, Qiu B, Zhang G (2011) The interaction of salinity and chromium in the influence of barley growth and oxidative stress. Plant Soil Environ 57:153–159
Appenroth KJ, Krech K, Keresztes A, Fischer W, Koloczek H (2010) Effects of nickel on the chloroplasts of the duckweeds Spirodela polyrhiza and Lemna minor L. and their possible use in biomonitoring and phytoremediation. Chemosphere. 78(3):216–223
Axtell NR, Sternberg SP, Claussen K (2003) Lead and nickel removal using Microspora and Lemna minor. Bioresour Technol 89(1):41–48
Balen EV, van de Geijn SC, Desmet GM (1980) Autoradiographic evidence for the incorporation of cadmium into calcium oxalate crystals (heavy metal)[tomatoes]. Zeitschrift fuer Pflanzenphysiologie (Germany, FR)
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254
Brooks RR (1998) Plants that hyperaccumulate heavy metals, their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining
Cunningham SD, Ow DW (1996) Promises and prospects of phytoremediation. Plant Physiol 110(3):715
Dembitsky VM, Rezanka T (2003) Natural occurrence of arseno compounds in plants, lichens, fungi, algal species, and microorganisms. Plant Sci 165(6):1177–1192
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. JXB 32(1):93–101
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135(1):1–9
Diwan H, Ahmad A, Iqbal M (2012) Chromium-induced alterations in photosynthesis and associated attributes in Indian mustard. J Environ Biol 33(2):239
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS (2013) Morphological, physiological and biochemical responses of different plant species to Cd stress. Int J Chem Biochem Sci 3:53–60
Ghani A (2011) Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egypt. Acad J Biol Sci 2(1):9–15
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J Energy Environ 6(4):18
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32(6):481–494
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22(2):1534–1544
Haouari CC, Nasraoui AH, Bouthour D, Houda MD, Daieb CB, Mnai J, Gouia H (2012) Response of tomato (Solanum lycopersicon) to cadmium toxicity: growth, element uptake, chlorophyll content and photosynthesis rate. African J Plant Sci 6(1):001–007
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45(1):62–69
Ikhuoria EU, Okieimen FE (2000) Scavenging cadmium, copper, lead, nickel and zinc ions from aqueous solution by modified cellulosic sorbent. Int J Environ Sci 57(4):401–409
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62(5):648–662
Júnior CA, de Sousa BH, Galazzi RM, Koolen HH, Gozzo FC, Arruda MA (2015) Evaluation of proteome alterations induced by cadmium stress in sunflower (Helianthus annuus L.) cultures. Ecotoxicol Environ Saf 119:170–177
Kang JW (2014) Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol Lett 36(6):1129–1139
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241(4):847–860
Khlifi R, Hamza-Chaffai A (2010) Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: a review. Toxicol Appl Pharmacol 248(2):71–88
Manohar S, Jadia C, Fulekar MH (2007) Impact of ganesh idol immersion on water quality. Indian J Environ Prot 27(3):216
Metzner H, Rau H, Senger H (1965) Untersuchungenzursynchronisierbaketieinzel- nerpigmentmangel-mutation von chlorella. Planta 65:186–194
Mishra VK, Tripathi BD (2008) Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour Technol 99(15):7091–7097
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Monalisa M, Kumar PH (2013) Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (Vigna radiata L. wilczek. var k-851) during seedling growth. J. stress physiol. biochem. 9(2)
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Najeeb U, Ahmad W, Zia MH, Zaffar M, ZhouW. (2017) Enhancing the lead phytostabilization in wetland plant Juncus effusus L. through somaclonal manipulation and EDTA enrichment. Arab J Chem 10:S3310–S3317
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186(1):565–574
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Pandey VC, Singh JS, Singh RP, Singh N, Yunus M (2011) Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour Conserv Recycl 55(9-10):819–835
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54:45
Prasad MNV (2003) Phytoremediation of metal-polluted ecosystems: hype for commercialization. Russ J Plant Physiol 50(5):686–701
Prasad R, Shivay YS (2017) Oxalic acid/oxylates in plants: from self-defence to phytoremediation. Curr Sci 112:1665–1667
Rodríguez MC, Barsanti L, Passarelli V, Evangelista V et al (2007) Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonas reinhardtii. Environ Res 105(2):234–239
Sallah-Ud-Din R, Farid M, Saeed R, Ali S, Rizwan M, Tauqeer HM, Bukhari SAH (2017) Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. ESPR. 24:17669–17678
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753
Singh D, Tiwari A, Gupta R (2012) Phytoremediation of lead from wastewater using aquatic plants. J Adv Agric Technol 8(1):1–11
Sood A, Uniyal PL, Prasanna R, Ahluwalia AS (2012) Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 41:122–137
Wang W (1990) Literature review on duckweed toxicity testing. Environ Res 52(1):7–22
Wolińska A, Stępniewska Z, Włosek R (2013) The influence of old leather tannery district on chromium contamination of soils, water and plants. J Nat Sci 5(02):253
Ye WL, Khan MA, McGrath SP, Zhao FJ (2011) Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ Pollut 159(12):3739–3743
Zaheer IE, Ali S, Rizwan M, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Saf 120:310–317
Zayed A, Gowthaman S, Terry N (1998) Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J Environ Qual 27(3):715–721
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35(5):785–791
Author information
Authors and Affiliations
Contributions
Umer Hayat and Shafuq Abbas carried out the experiment. Umer Hayat wrote the manuscript and supervised the project. Shafuq Abbas contributed to the analysis of the results. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This data is original and the manuscript is not submitted to any other journal. It is a plagiarism free manuscript.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
This study is pure and new and journals has full rights to publish this research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayat, U., Abbas, S. Oxalic acid-assisted phytoextraction of heavy metal contaminated wastewater through Lemna minor L.. Environ Sci Pollut Res 30, 103972–103982 (2023). https://doi.org/10.1007/s11356-023-29547-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29547-w