Abstract
This study assessed the emissions of gaseous pollutants and particle size distributed water-soluble organics (WSO) from a diesel vehicle fuelled with ultralow sulphur diesel (B0) and 10 (B10), 20 (B20), and 30% (B30) biodiesel blends in a chassis dynamometer tested under transient mode. Particulate emission sampling was carried out in an ultraviolet (UV) test chamber using a 10-stage impactor. Samples were grouped into three size fractions and analysed by gas chromatography-mass spectrometry. Increasing the biofuel ratio up to 30% in the fuel reduced WSO emissions by 20.9% in comparison with conventional diesel. Organic acids accounted for 82–89% of WSO in all tested fuels. Dicarboxylic acids were the most abundant compound class, followed by hydroxy, aromatic, and linear alkanoic acids. Correlations between compounds demonstrated that adding biodiesel to diesel fuel reduces the emissions of nitrogen oxides (NOx), benzene, toluene, ethylbenzene and xylenes (BTEX), methane (CH4), total and nonmethane hydrocarbons (THC and NMHC), and dicarboxylic and hydroxy acids, but increases emissions of carbon dioxide (CO2) and alkanoic and aromatic acids. Emissions of dicarboxylic and hydroxy acids were strongly correlated with the biodiesel content. WSO emissions of coarse and fine (1.0–10 μm) particles decreased with the increasing biofuel content in fuel blend. The total share of ultrafine (0.18–1.0 μm) and nanoparticles (< 0.18 μm) increased in WSOs emissions from B20 and B30 blends, when compared with petrodiesel. The biodiesel content also affected the chemical profile of WSO size fractions.
Similar content being viewed by others
Introduction
Biodiesel has received increasing attention worldwide as an alternative fuel in vehicle engines due to the scarcity of conventional fossil fuels, energy security concerns, and environmental issues. This interest results from its renewable origin, biodegradability, lower greenhouse gas (GHG) emissions, reduction in harmful exhaust emissions, low toxicity, and health concerns (Damanik et al. 2018; Živković and Veljković 2018; Verma et al. 2019).
Biodiesel consists of long-chain fatty acid alkyl esters and is typically produced from vegetable oils or animal fats through transesterification reaction of lipids with short-chain monohydric alcohol in the presence of alkali, acid, or enzyme catalysts (Mathew et al. 2021; Moser 2009). More than 350 feedstocks have been identified for production of biodiesel (Atabani et al., 2012), and among them, according to Souza et al. (2018), soybean, rapeseed, and palm oils are the main sources. Rapeseed, palm, soybean, and sunflower oils are the most used in the European Union (Bockey 2019). Soybean is the main raw material in the USA and the Brazilian biodiesel sector (da Silva et al. 2019; Hoekman 2009; Kumar et al. 2013; Meira et al. 2015).
Diesel engines are among the major sources of carbon monoxide (CO), nitrogen oxides (NOx), hydrocarbons (HC), and particulate matter (PM) (Ghazali et al. 2015 and references therein). When compared to standard diesel, the reduction in polycyclic aromatic hydrocarbons (PAHs) and near zero sulphur content, as well as oxygen enrichment and increase in cetane number in biodiesel, can have a positive effect on combustion characteristics and/or on engine exhaust emissions (Amaral et al. 2017; Ashraful et al. 2015; Ferreira et al. 2008; Tsai et al. 2010; Wang et al. 2016). Biodiesel, regardless of generation, used directly without modification or blended with conventional diesel in any proportions, benefits the physicochemical properties of the fuel and reduces exhaust emissions of diesel engines (Alptekin et al. 2015; Najafi 2018). Chemical compositions of biodiesel differ upon their origin and lead to variation in their properties and performance in terms of emission characteristics (Ghazali et al. 2015; Kumar et al. 2013). In general, most biodiesel blends result in a significant decrease in CO and total unburned HC emissions and significant increase in NOx emissions (Ghazali et al., 2015; Hassan and Rahman 2017; Palani et al. 2022). Nevertheless, the use of biodiesel is not always favourable in terms of PM emissions, which may also depend on the refinement degree of fuel and after treatment technologies (Kontses et al. 2019; Wang et al. 2016 and references therein).
PM emissions have been always the main concern of manufactures due to their effect on the performance of the engines, environment, and human health. In fact, vehicular exhaust particles are related to adverse health effects such as airway inflammation (Ghio et al. 2012), vascular dysfunction (Mills et al. 2005), developmental toxicity (Ema et al. 2013), neuroinflammation (Levesque et al. 2011), and respiratory mortality (Atkinson et al. 2016), among others. Biological assays demonstrated that addition of biodiesel to diesel fuels can reduce PM emissions but not necessarily the adverse health outcomes (Fukagawa et al. 2013; Mehus 2015).
Particulate matter from vehicle exhausts contains a variety of chemical constituents such as elemental carbon (EC), organic carbon (OC), trace elements, metal oxides, a wide range of hydrocarbons (HC), organic oxygenated compounds, sulphur compounds, and other species (Cheung et al. 2010; Wang et al. 2016). These particles differ in size, composition, and solubility, which can directly influence their possible toxic properties. Its physical and chemical characteristics also depend on different parameters such as fuel properties, engine type, operating conditions, fuel injection mode, vehicle age and type, and after-treatment technology (Corrêa et al. 2021; Kontses et al., 2019; Verma et al. 2019).
Numerous studies have been focused on emissions from vehicles with traditional or alternative fuels. There are some recent literature reviews that summarised investigations on the performance and exhaust emission characteristics of biodiesel blends in diesel engines, including studies on the respective effects on human health (Damanik et al., 2018; Ghazali et al. 2015; Hasan and Rahman 2017; Palani et al. 2022; Verma et al. 2019). However, studies mentioned in these reviews discuss the impact of biodiesels and their blends on exhaust emissions of regulated pollutants (CO, HC, NOx, and PM) by environmental legislation in several countries. On the other hand, among unregulated pollutants, carbonyl compounds, BTEX (benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene), EC, OC, total carbon (TC = EC + OC), and PAHs were the most characterised (Amaral et al. 2017; Bakeas and Karavalakis 2013; Bório et al. 2019; Borrás et al. 2009; Casal et al. 2014; Chiang et al. 2012; Corrêa et al. 2021; Corrêa and Arbilla 2006; Ferreira et al. 2008; Karavalakis et al. 2009, 2010a; Li et al. 2018; Lim et al. 2014; Martins et al. 2012; Wang et al. 2021). Cheung et al. (2010) conducted a more detailed study in a chassis dynamometer and determined emission factors (EFs) of particulate trace elements, metals, and solvent-extractable organic species (PAHs, hopanes, steranes, n-alkanes, and organic acids) from diesel or biodiesel passenger vehicles. Ghadikolaei et al. (2019) studied the PM chemical composition (TC, OC, EC, water-soluble organic carbon (WSOC), inorganic ions, metals, and elements) from a diesel engine fuelled with ternary fuel (diesel-biodiesel-ethanol) in different fuelling modes. However, few studies have investigated the chemical composition of biodiesel exhaust particulate matter in a size-segregated mode. Lin et al. (2008a, b) have assessed particle size distributions of PM and PAHs emitted from heavy-duty diesel engines fuelled with biodiesel blends. Rocha and Corrêa (2018) have characterised metals in coarse, micrometric, ultrafine, and nanoparticles in exhaust emissions from a typical diesel engine used by buses and trucks in Brazil fuelled with diesel-biodiesel blends. Size-segregated PAHs, nitro-PAHs, and alkyl-PAHs in emissions from diesel-biodiesel blends were studied by Corrêa et al. (2021). The volatile fraction (VOF) and the EC content in PM emissions were analysed according to different particle sizes, and the subsequent effect of oxygen content in biodiesel on the size-resolved characteristics of PM emissions was discussed by He et al. (2017). Recently, Li et al. (2021) measured the exhaust emissions of organic acids from gasoline, diesel, and liquefied petroleum gas (LPG) vehicles in China. The tests were performed in a dynamometer system using an iodide-adduct time-of-flight chemical ionisation mass spectrometer. Fuel-based and mileage-based emission factors of C1–C5 carboxylic acids, hydrogen cyanide, and isocyanic acid were obtained. It was subsequently concluded that emissions of carboxylic acids from diesel vehicles are much higher than those from gasoline vehicles.
WSOC can reach approximately 27–83% of the organic carbon mass of aerosols (Yu et al. 2004 and references therein) and is primarily emitted from combustion of biomass and fossil fuels, or secondarily formed from oxidation of VOCs, aromatic compounds, and high molecular weight hydrocarbons with anthropogenic or biogenic origin (Du et al., 2014; Park et al., 2015). WSOC affect the formation and physical properties of clouds, Earth’s radiative balance, and atmospheric chemistry with implications for regional and global climate change (Duarte et al., 2015, 2019; Niu et al. 2022; Tang et al., 2020). Moreover, due to their toxicity, particle-bound WSOC has been associated with human cardio-respiratory diseases (Cheung et al. 2009; Ramgolam et al. 2009). Water-soluble organic acids (WSOAs) are an important fraction of WSOC and have been comprehensively investigated across the world in different outdoor environments (Tang et al. 2020 and references therein). However, they have been sparsely documented in emissions from diesel/biodiesel combustion. Due to their high water solubility, carboxylic acids can potentially modify the hygroscopic properties of atmospheric particles, including their ambient size, and play an important role on cloud condensation nuclei activity (Kumar et al. 2003; Xu et al. 2010; Yu et al. 2000; Zhang et al. 2004). WSOAs are also relevant components of the secondary organic aerosol (SOA) and can contribute to the understanding of its chemical composition, sources, and formation mechanisms. Vehicle emissions were pointed out as an important source of WSOAs in urban environments (Bao and Sakamoto 2009; Bock et al. 2017; Kawamura et al. 1987, 2000; Li et al. 2021). However, dynamometer studies focused on the chemical composition of the water-soluble organic matter in exhaust emissions from engines fuelled with diesel, biodiesel, and biodiesel blends are, as far as we know, very scarce (Bock et al. 2017; Kawamura and Kaplan 1987; Kawamura et al., 2000). Also, most of these studies have assessed the concentrations of a limited number of organic water-soluble species in emissions mainly from older vehicle engines. Therefore, a more detailed characterisation of combustion emissions (e.g., emission factors) from diesel/biofuel engines is required for efficient and sustainable utilisation of biodiesel as an alternative to conventional fuel, as well as for source apportionment and for environmental control strategies. Moreover, the determination of the detailed molecular composition of WSOC has relevance for understanding the impacts of traffic emissions on physicochemical transformations in the atmosphere, ecosystems, and climate, as well as the associated effects on air quality and health. In this context, this paper presents results from a chassis dynamometer study conducted to determinate exhaust emissions from a diesel engine, using four binary mixtures of fossil diesel with biodiesel (0, 10, 15, 20, and 30%). It is important to underline that combustion conditions were kept the same between tests, which allowed evaluating the influence of the proportion of biodiesel in the fuel on the amount and chemical composition of exhaust engine emissions. The detailed particle size-segregated emission profiles of this study, with special focus on the water-soluble organic fraction, can be useful for source apportionment studies.
Materials and methods
Experimental facility
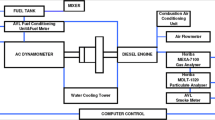
The tests were conducted with a chassis dynamometer belonging to the Lactec Laboratory, Institute of Technology for Development, Curitiba, Brazil. A light commercial vehicle Renault Master 2.5 L, 16 valve diesel engine with 84 kW at 3500 rpm, from 2012 with 71,000 km, was tested in the dynamometer system. A Horiba Mexa 7200 (CO, HC, NOx, CO2, CH4) bench analyser and 7500 DEGR (CO, HC, NOx, CO2, O2) were used to quantify the gaseous compounds. Total hydrocarbons (THC) were determined by flame ionisation detection (model FIA-720, 0–50 ppmC), carbon monoxide (CO) and carbon dioxide (CO2) were quantified by non-dispersive infrared spectrometry (model AIA-721A, 0–200 ppm, and AIA-722, 0–2.0% v/v, respectively), and nitrogen oxides (NOx) were measured by chemiluminescence (model CLA-720A, 0–50 ppm).
The vehicle engine was equipped with an after-treatment system that included a diesel oxidation catalyst (DOC) and an exhaust gas recirculation valve (EGR). This engine is a standard type widely used in the vehicle fleet of Brazilian towns and cities, such as school transport, company vans, and community transport. A constant volume sampler (CVS) was used to dilute the exhaust emissions with ambient air, which was pre-cleaned from particles through a quartz filter. Emission tests were performed in transient mode, according to the Brazilian standard ABNT NBR 6601 (2021), similar to the US FTP-75 standard, and included three phases (cold start transition phase, stabilised phase, and hot start transient phase).
The diluted emissions, after the measurement of the primary pollutants, were transferred to a reaction chamber, built with 5 mil Teflon FEP, inert to the exhaust gases and permeable to UV rays. The chamber had a volume of 3.9 m3, and included an aluminum support frame, resulting in dimensions of approximately 1.19 m × 1.19 m × 2.78 m. Both the sides and the bottom of the chamber were covered with a reflective shield to increase the incident radiation, as well as to prevent people from being exposed to UV rays. The simulation of sunlight was based on the work of Barnes and Rudzinki (2006), involving the use of 8 fluorescent lamps (Philips TUV30W G30T8 and UV–C and Bravo F30T8/BL UV-A) placed at the top of the appliance that emit UV radiation with peak at 254 nm and 365 nm, respectively. A 100-mm fan was placed inside the chamber (at the bottom) to achieve a better homogenisation of the gases.
Fuels and sampling
The tested fuels included standard reference S10 (10 mg kg−1 of sulphur) pure diesel (B0) and four blends with soybean biodiesel B100 in the following proportions: B10, 10% volume of biodiesel, with a density of 835.0 kg m−3; B20, 20% biodiesel, with a density of 840.0 kg m−3; and B30, 30% biodiesel, with a density of 845.0 kg m−3.
Sampling of PM exhaust emissions was carried out using a 10-stage MSP nano MOUDI (micro-orifice uniform deposit impactor) II model 120R cascade impactor. In each stage, < 0.025-mm aluminium disks (MSP Corp. Subst Foil; 0100-96-0573A-X) with 47 mm diameter were used as substrates. The samples were collected from the Teflon fluorinated ethylene propylene (FEP) chamber after UV irradiance for 1 h. Accordingly, PM represents a mixture of non-reactive primary material emitted from the exhaust pipe and SOA formed in the reaction chamber. The sampling flow and the pressure drop were 30 L min−1 and 40 kPa, respectively. Particles were collected according to the following cut-point diameters: 10, 5.6, 3.2, 1.8, 1.0, 0.56, 0.32, 0.18, 0.10, and 0.056 μm. Samples were refrigerated (− 20 °C) immediately after the chassis dynamometer experiments.
An activated charcoal cartridge (Supelco ORBO 32 400/200 mg) was used for BTEX collection at a flow rate of 1.5 L min−1. The cartridges have 2 beds, a main one of 400 mg and a secondary one of 200 mg. Both beds were extracted, treated and chemically analysed in the same way. When the secondary bed has more than 5% of the total mass of analytes contained in the main bed, the sample is discarded, but this did not occur in any of the tests in this work. One cartridge was used for each phase of the standard protocol and another cartridge for the collection of dilution air. The contents of each cartridge were transferred to a 2-mL vial and added to 1000 μL of dichloromethane at − 20 °C. The flasks were capped with septum caps, placed in an ultrasonic bath for 20 min, and then allowed to rest for 1 h (Correa et al., 2012; Corrêa and Arbilla, 2007, 2006; Daemme, 2016a; Daemme, 2016b; Garcia et al., 2013; Macedo et al., 2017; Martins et al., 2016, 2007).
Carbonyl emissions (RCHO) were sampled using impingers with 2,4-dinitrophenylhydrazine acid solution (2,4-DNPH) following the guidelines of ABNT NBR 12026 (2021).
Chemical analyses
The PM samples were grouped into three fractions to obtain enough mass for ulterior chemical analysis, as follows: coarse and fine fraction (1.0–10 μm), ultrafine particles (0.18–1.0 μm), and nanoparticles (< 0.18 μm).
Each filter set was ultrasonically extracted with 10 mL of ultra-pure water for 30 min with a 5-min stop in the middle. The extract was filtrated through a 13-mm PVDF syringe filter with 0.2-μm pore size (Whatman™, Buckinghamshire, United Kingdom) to remove any insoluble particles. The water extract was concentrated using a Turbo Vap® II concentrator (Biotage) and dried under a nitrogen stream.
Prior to speciation, oxygenated compounds were converted into the corresponding trimethylsilyl derivatives by addition of N,O-bis (trimethylsilyl) trifluoroacetamide (BSTFA):trimethylchlorosilane (TMCS) 99:1 (Supelco) and pyridine containing 2 internal standards: 1-chlorohexadecane (Merck) and tetracosane-d50 (Aldrich). The reaction mixture was heated in an oven at 70 °C for 3 h. The silylated derivatives were analysed by gas chromatography-mass spectrometry (GC-MS) from Thermo Scientific (Trace Ultra, quadrupole DSQ II) equipped with a split/splitless injector and a fused silica capillary column (TRB-5MS, 60 m × 0.25 mm × 0.25 μm). Helium was used as carrier gas at a constant flow of 1.2 mL min−1. The oven temperature program was as follows: 60 °C (1 min); 60–150 °C (10 °C min−1), 150–290 °C (5 °C min−1), and 290 °C (30 min). The derivatised extracts were analysed in both full scan and selected ion monitoring (SIM) modes. The acquisition mode was electronic impact at 70 eV, and the scanned masses ranged from 50 to 850 m/z. The GC–MS calibration was performed via injection of authentic standards in six concentration levels (5–50 ppm). Standards and samples were both co-injected with internal standards. All chemicals used were of analytical reagent grade from Sigma-Aldrich. The detection limit (LD) and quantification limit (LQ) for WSOAs varied from 0.03 to 0.70 and from 0.07 to 20.8 ppm, respectively, depending on the compound. Generally, the extraction recovery ranged between 77 and 99%. Extraction recovery tests were carried out with five blank filters, spiked with known quantities of the target analytes (standards). The impregnated filters were subsequently extracted and analysed following the procedures described above. The recovery was calculated as the ratio between the concentration of the standard determined after and before the extraction, expressed in percentage. EFs of the exhaust emission were expressed in a distance-based approach.
The BTEX chemical analyses were performed by GC-MS on a Varian 450GC 220MS chromatograph using a VF-5MS column (30 m, 0.25 mm, and 0.25 μm). Injections of 1.0 μL of sample were conducted at 200 °C, with a split ratio of 1:4, using helium 5.0 as carrier gas at 2.0 mL min−1. The initial column temperature was 40 °C, which was maintained for 3 min, and then followed by a heating rate of 15 °C min−1 up to 200 °C, which was held for 6 min. The temperatures of the ion trap, manifold, and transfer line were 150 °C, 40 °C, and 180 °C, respectively. The MS detector monitored ions from 72 to 79, 89 to 93, 101 to 107, and 119 to 121 (m/z) (Correa et al., 2012; Corrêa and Arbilla, 2007, 2006; Daemme, 2016a; Daemme, 2016b; Garcia et al., 2013; Macedo et al., 2017; Martins et al., 2016, 2007). The calibration was performed with a standard BTEX solution (Supelco EPA TO-1 Mix 1A) by external standardisation with concentrations of 0.10, 0.20, 0.50, 1.00, 2.00, and 4.00 ng mL−1, as an acceptance criterion of the analytical curve determination coefficients higher than 0.99. The calculated quantification limit for each BTEX compound was 5.6 pg mL−1, which corresponds to a concentration of 1.0 mg m-3 in the gas phase (Correa et al. 2012). All the measurements were within the analytical curves for all the samples and no dilution was necessary.
Carbonyl chemical analyses were performed by high performance liquid chromatography (HPLC) on an Agilent LC1200 with a G1314D detector at 365 nm. A volume of 20 μL was injected using a ZORBAX ODS column (25 cm × 4.6 mm × 5.0 μm) maintained at 35 °C. The mobile phase employed was 65% acetonitrile and 35% water at a constant flow of 1.0 mL min−1. The analytical curves were prepared by means of two types of standards, a Supelco 47650-U Mix and CRM47651 Mix.
Data treatment
R language version 3.3.1 (Core Team 2016) was used for the processing of the study data. Correlations were calculated with 95% significance.
Results and discussion
Exhaust emissions of primary regulated and unregulated pollutants
Addition of biodiesel to conventional diesel led to significant reductions in emissions of BTEX, THC, and nonmethane hydrocarbons (NMHC) (Table 1). The high oxygen content in biodiesel promotes a more complete combustion, which reduces unburned hydrocarbon emissions (Ghazali et al., 2015; Hassan and Rahman 2017). Regarding regulated pollutants, emission levels of CO and NOx, for all tested fuels, were greatly above the Euro 5 and Euro 6 emission limits. PM emissions also exceeded both limits (Table 1).
The effect of oxygenated fuel blends on the NOx emission profiles have been investigated in numerous studies. However, the results from literature are complex and inconclusive. Many studies reported increased NOx emissions with increasing biofuel content (Ghazali et al. 2015 and references therein). In contrast, a reduction in NOx emissions with the use of biodiesel was observed in other studies (Abu-Hamdeh and Alnefaie 2015; Armas et al. 2010; Hoekman and Robbins 2012; Puhan et al. 2005; Serrano et al. 2015; Qi et al. 2009; Zhang et al., 2008). According to Ghazali et al. (2015), NOx emissions are dependent on the fuel’s oxygen content. Higher oxygen contents and cetane number of biodiesel contribute to higher combustion temperature and therefore favour complete combustion and NOx formation. Also, high NOx emissions of biodiesel blends have been related to injection timing, driving cycle, and fuel proprieties (Karavalakis et al. 2010b; Lim et al. 2014). In this study, a slight decrease in NOx emissions was observed. This effect may be associated with the fact that the engine was equipped with an exhaust gas recirculation valve (EGR). The application of an EGR decriases the cylinder temperature (due to introduction of diluent gas of high specific heat) and reduces the oxygen content in the cylinder (Chuepeng et al. 2007; Zhang et al. 2008).
Figure 1 presents a Pearson’s correlation matrix between biodiesel content, consumption, regulated/unregulated pollutants, and the fraction of WSO. A cohesive data set was observed between the variables THC, NMHC, NOx, BTEX, and WSO, all with positive correlations (Fig. 1). Other strong positive correlations were registered between CH4 with WSO, THC, and NMHC. Also, CO2 presented very strong negative correlations with THC, NMHC, WSO, and CH4. The biodiesel content reduces the emissions of WSO, THC, NMHC, CH4, and BTEX to a greater degree, but indicates an increase in CO2. Fuel consumption was little influenced by biodiesel content but had a strong negative correlation with carbonyl compounds (RCHO).
Exhaust emissions of water-soluble organics and size fractions
A decrease in emissions of WSO with increasing biofuel content in diesel was observed (Fig. 2). The reductions were 9.1, 14.9, and 20.9% for B10, B20, and B30 blends, respectively, when compared to diesel engine emissions. Considering that the operating conditions during chassis dynamometer testing were very uniform, this reduction can be related to the increase in the percentage of biofuel in the biodiesel blend. This assumption is confirmed by a very strong negative correlation between the biodiesel content and the emissions of WSO (Fig. 1).
Most constituents of fossil diesel are saturated hydrocarbons (primarily alkanes, including n, iso, and cycloalkanes). Aromatic compounds (naphthalenes and alkylbenzenes) are present in an even lesser amount (Al Qubeissi 2015). In the case of complete combustion, all organic matter of the fuel should be converted into CO2 and water. Alkanes are the most reduced compounds and, under combustion, are initially oxidised to alcohols, then to carbonyl compounds, then to carboxylic acids, then to esters, and finally to CO2 (Mkoma et al. 2014).
Soybean biodiesel mainly consists of methyl esters from linoleic, oleic, and palmitic acids, together with linolenic and stearic acids in lesser amount (Lam et al. 2010). Furthermore, the cetane number of biodiesels is higher than that of petrodiesel. Thus, the addition of biodiesel to fossil diesel decreases the share of saturated hydrocarbons and increases the cetane number and oxygen content in the fuel. According to Wang et al. (2021), the high cetane number and oxygen content of biodiesel contribute to a more complete combustion in the diesel engine and could explain the decrease of WSO emissions detected in the present study.
In the literature, there is no information on WSO emissions from biodiesel combustion. Scarce information was given about WSOC emissions. Cheung et al. (2009) conducted emission tests in a dynamometer facility for light-duty vehicles operated with petrodiesel (with 10 or 50 ppm of sulphur) and neat soybean biodiesel. Significant high WSOC emissions of 0.91, 1.48, and 1.42 mg km−1 were measured for a Honda Accord (Euro 4, diesel S10, capacity of 2.2 L), VW Golf TDi (Euro 2, 100% soybean biodiesel, capacity of 1.9 L), and VW Golf TDi (Euro 1, diesel S50, capacity of 1.9 L), respectively. A substantially lower emission (0.10 mg km−1) of WSOC was reported for the Honda Accord (Euro 4+) equipped with diesel particulate filter (DPF). The authors mentioned that to convert WSOC to water-soluble organic matter (WSOM), a factor of 2 can be used. Ghadikolaei et al. (2019) have investigated the effects of different fuelling modes on the composition of PM emissions under different operating conditions. An increase in WSOC, metals, and elements was observed, but a reduction in TC, EC, OC, and water-soluble inorganic carbon (WSIC) for blended fuel, when compared with diesel, was simultaneously registered. Worth noting that the amount, physical proprieties, and chemical composition of exhaust emissions vary greatly depending on different factors such as type of engine, fuel, operation conditions, and various technologies applied on the diesel engines and driving cycles, among others. So, the comparison of emissions from different studies is only adequate to indicate possible trends, main differences, and order of magnitude of emissions. Regarding the size profile, the share of coarse and fine particles (C&F) in WSO emissions decreased with increasing percentages of biofuel in diesel blends.
Chemical composition of WSO emissions
Significant differences were also observed in the chemical composition of emissions according to size distribution. As it turned out in this study, the increase of biofuel content in diesel led to a decrease of WSO emissions. Organic acids were the major compound class, accounting for 82–89% of WSO. Following a similar behaviour of WSO, their ΣEFs decreased from 18.8 (B0) to 14.6 (B30) μg km−1 with the increase of biofuel content in diesel (Fig. 3).
Rather variable emissions of organic acids were observed in organic extracts from dynamometer tests by Cheung et al. (2010). In their study, ∑EFs of organic acids (C9–C29) were 1.04 ± 3.74 μg km−1, 342 ± 43 μg km−1, and 5240 ± 355 μg km−1 for diesel S10 (Euro 4, 2.2 L engine), 100% soybean biodiesel (Euro 2, 1.9 L), and diesel S50 (Euro 1, 1.9 L) vehicle engines, respectively. In the present study, significantly lower emissions were observed for glycerol (1.45 – 1.87 μg km-1) and polyethylene glycols (0.179 – 0.535 μg km-1) with no trend towards the percentage of biodiesel in fuels. No clear relationship was observed between the size distributed organic acids and biofuel content (Fig. 3). However, increased emissions of organic acids and glycerol with increased particle size were registered for the combustion of standard diesel (B0). Coarse and fine emissions of acids were ca. two times higher when compared with nanoparticles for B0, B10, and B20 blends. Nevertheless, the size distribution of emissions from B30 was fairly homogeneous. Ultrafine and coarse and fine fractions presented comparable acid emissions for B20, while the B10 blend had similar values for ultrafine and nanoparticles.
Six classes of organic acids were determined in WSO emissions (Fig. 4). Carboxylic acids, including alkanoic, dicarboxylic, aromatic, and hydroxy acids, were the most abundant. Their emission profile was very similar for B0, B10, and B20. However, in the case of the B30 experiment, aromatic acids practically doubled their share for WSO exhaust emissions (Fig. 4). Emissions of hydroxy acids were reduced from 32–36% to 24%, respectively. These results suggest that the addition of 30% biodiesel significantly affected the chemical composition of organic acid classes, while no notable changes were registered for other fuels tested.
Previous studies have pointed out that organic acids can be directly emitted from diesel engines as a result of combustion processes of the fuel (Bock et al. 2017; Kawamura et al. 2000). It was also suggested that C1–C10 organic acids can be formed in the atmosphere by photochemical oxidation of unsaturated hydrocarbons and aldehydes, which are emitted from motor exhaust in amounts of several orders of magnitude higher than organic acids (Kawamura et al. 2000).
Size-segregated exhaust emissions of different classes of carboxylic acids are shown in Fig. 5. Dicarboxylic and hydroxy acids were the most abundant with ∑EFs in the ranges 8.78–6.17 and 6.72–3.49 μg km−1, respectively. Their emissions were significantly decreased with rising biodiesel content in the blends. The reduction in emissions was 30% for dicarboxylic acids and 48% for hydroxy acids when comparing conventional diesel with the B30 biodiesel blend. Dicarboxylic acids were very strongly negatively correlated with biodiesel content in the fuel and positively correlated with hydroxy acids and with WSO (Fig. 6).
Likewise, hydroxy acids presented very strong negative correlations with biofuel content and positive correlations with WSO. This suggests that addition of biofuel in biodiesel blends leads to significant decreases in exhaust emissions of diacids and hydroxy acids, which possibly originate via similar pathways during the combustion process. Aromatic acids showed a different pattern (Fig. 5). Their ∑EFs from engine fuelled with B0, B10, and B20 were comparable and ranged between 1.63 and 2.06 μg km−1. However, an increase in emissions (3.39 μg km−1) was observed for B30.
Also, aromatic acids exhibited strong negative correlation with other acids, strong negative correlation with polyethylene glycols, moderate negative correlations with hydroxy acids, and strong positive correlation with glycerol (Fig. 6). Emissions of alkanoic acids from B0 and B10 tests were lower (0.87–1.11 μg km−1) when compared with B20 and B30 (1.42–1.76 μg km−1). Moderate correlations were observed for alkanoic acids and dicarboxylic acids, amino acids, hydroxy acids, glycerol, and other compounds (Fig. 6).
Regarding the size distribution, a percentage reduction in emissions of dicarboxylic acids, hydroxy acids, and alkanoic acids in coarse and fine particles versus ultrafine and nanoparticles was registered (Fig. 5). Aromatic acids presented an irregular size distribution profile, registering significant increases, up to 49%, in emissions of nanometric particles, from B30 versus 18% from B0.
Emission factors of carboxylic acids
The individual size-segregated emission factors for dicarboxylic, aromatic, and hydroxy acids are presented in Table 2. Emissions of alkanoic acids and other compound classes, which had a minor contribution to WSO, are given in the Supporting Information (Table S5). The molecular structures of compounds listed in Tables 2 and S5 are presented in Figs. S1A, S1B, S1C, and S1D in the Supporting Information. Among dicarboxylic acids, oxalic and succinic acids were the most abundant in emissions of all tested fuels, whose EFs (sum of all size fractions) ranged between 3.79–6.25 and 1.04–2.04 μg km−1, respectively (Fig. S2). Regarding the size distribution, no trends were observed for emissions of oxalic acid. However, size-segregated EFs of other acids were generally higher for nanometric particles than for other size fractions (Table 2). Analysing the correlations between dicarboxylic acids (Table S1), it was observed a very strong positive relationship between malic-pimelic (r = 0.92), malic-thapsic (r = 0.90), pimelic-suberic (r = 0.90), pimelic-thapsic (r = 0.99), and suberic-azelaic (r = 0.92) acids. Also, oxalic-adipic (r = 0.75), succinic-glutaric (r = 0.70), malic-suberic (r = 0.83), malic-azelaic (r = 0.84), adipic-azelaic (r = 0.77), pimelic-azelaic (r = 0.75), and suberic-thapsic (r = 0.84) acids were strongly positively correlated. Dicarboxylic acids are highly oxidised compounds and usually show high water solubility. These organic acids extensively participate in various chemical and physical processes in the atmosphere and appear as a relevant part of WSOAs. Diacids mostly derive from secondary production (Kawamura and Yasui, 2005). However, Kawamura and Kaplan (1987) and Bock et al. (2017) argued that diesel engines are the primary sources of both gas and particle phase dicarboxylic acids. Kawamura and Kaplan (1987) analysed gas and particle phase concentrations of C2–C10 dicarboxylic acids emitted from a diesel engine without after-treatment system. In their study, oxalic, maleic, and methylmaleic diacids were detected as major species in exhaust emissions. It was suggested that incomplete combustion of cyclic olefines probably produces saturated diacids, which may be further oxidised to oxalic acid during combustion in motor exhausts. Azelaic acid is mostly formed by the photooxidation of biogenic unsaturated aliphatic acids (Kawamura and Kaplan 1987). However, this diacid was also detected in exhaust emissions by Kawamura and Kaplan (1987). The authors proposed that normal mono- and dicarboxylic acids can be the combustion products of normal alkanes in fuels. In other investigation, vapour-phase, semi-volatile, and particle-phase organic compounds from motor vehicles have been studied in a roadway tunnel (Fraser et al. 1998). Particulate emission rates of 97.3, 33.6, 7.5, and 9.5 μg L−1 fuel were reported for succinic, glutaric, adipic, and azelaic acids, respectively. In our study, the EFs for these four acids ranged between 0.580–1.14, 0.094–0.143, 0.073–0.118, and 0.076–0.149 μg L−1 fuel, respectively. The significant difference in emissions observed between Fraser et al. (1998) and the present study can be related to several factors such as variations in test methodology (measurements in roadway tunnel vs dynamometer tests), motor characteristics (older vehicle fleet operated in roadway tunnel vs more modern car tested in our study), fuels (gasoline or diesel-powered vehicles in roadway tunnel vs diesel or biodiesel blend in our study), and analytical methods (organic solvent extractions vs water extraction), among others.
Seven hydroxy acids were detected in the WSO exhaust emissions (Table 2). Glycolic (hydroxyacetic) acid was the dominant species, accounting for 95% and 71–84% of all detected hydroxy acids in emissions of petrodiesel and diesel blends, respectively. Its emissions (all size fractions) ranged between 2.91 and 5.97 μg km-1 and presented a decrease with the addition of biofuel (Fig. S3). 3-Hydroxypropanoic acid was the second major hydroxy acid (EF = 0.21–0.58 μg km−1). With the exception of B10, emissions of this hydroxy acid from biodiesel blends were lower than that from conventional diesel. A very strong negative relationship (r = − 0.95) was observed between glycolic acid and biodiesel content (Table S2). 3-Hydroxypropanoic (hydracrylic) and 3-hydroxybutanoic acids were other abundant hydroxy acids. EFs of 3-hydroxybutanoic acid were also strongly negatively correlated (r = − 0.85) with biodiesel content. Additionally, positive and very strong (r = 0.96) and moderate (r = 0.68) correlations were observed between 3-hydroxybutanoic-glyceric and 3-hydroxypropanoic-3,4-dihydroxybutanoic acids, respectively. Except for 2-hydroxysebacic acid, which was not present in all the samples, a similar size distribution pattern was recorded for emissions from B0, B10, and B30 blends. The size-segregated EFs of hydroxy acids in nanometric particles were higher than those in ultrafine and coarse and fine size fractions. Worth noting that, up to date, there was no evidence that hydroxy acids can be primarily emitted from combustion of petrofuel/biodiesel in vehicle engines. Souza et al. (1999) reported the presence of glycolic and hydroxybutyric acids in a highly polluted urban atmosphere. The authors proposed biogenic emissions as possible sources for glycolic acid but have not established the origins of hydroxybutyric acid.
Benzoic acid was dominant among aromatic acids with EFs (all size fractions) between 1.86 and 3.33 μg km−1 (Fig. S4). Phthalic and terephthalic acids exhibited significant emissions with EFs between 31.3–85.9 and 9.95–89.9 ng km−1, respectively, and were the second most abundant species. Apart from cinnamic and syringic, all other acids were correlated with biodiesel content. Strong or moderate negative correlations were observed between biodiesel percentage and terephthalic (r = − 0.85), 4-hydroxybenzoic (r = − 0.80), vanillic (r = − 0.78), phthalic (r = − 0.62), and 4-hydroxybenzoic (r = − 0.47) acids, which generally experienced a reduction in exhaust emissions with the increase of biodiesel content (Table S4). In contrast, benzoic acid presented a positive moderate relationship (r = 0.67), recording very high emissions during the combustion of the B30 blend, when compared with petrodiesel. Likewise, high correlations were also observed between some of the aromatic acids. Terephthalic and vanillic acids showed very strong positive correlation (r = 0.98). 4-Hydoxybenzoic acid was strongly correlated with 3-hydroxybenzoic (r = 0.86) and phthalic (r = 0.74) acids. Strong positive correlations were observed between 3-hydroxybenzoic and phthalic (r = 0.84) and syringic (r = − 0.89) acids.
Emissions of aromatic acids, identified in the present work, did not show similar size-segregated distributions. EFs of benzoic and 4-hydroxybenzoic acids were higher for coarse and fine fraction and lower for nanoparticles during petrodiesel combustion (Table 2). However, exhaust emissions of these acids for the B30 blend were mostly found in nanoparticles, with the EFs for the coarse and fine size fraction being the lowest. An opposite pattern was observed for terephthalic and syringic acids, whose EFs were higher for nanoparticles from B0 and for the coarse and fine fraction from B30. It should be noted that an increase of biodiesel content led to an increase in emissions of ultrafine particles for benzoic acid and resulted in a reduction in emissions of coarse and fine particles of 4-hydroxybenzoic acid.
According to Kawamura et al. (1985, 2000) and Rogge et al. (1993), benzoic acid can be directly emitted from fossil fuel combustion. Phthalic and methylphthalic acids were documented among the major species in exhaust automobile emissions by Kawamura and Kaplan (1987). The authors proposed possible pathways for the formation of these acids from incomplete combustion of aromatic hydrocarbons (benzene, toluene, naphthalenes, and others) in car engines. Fraser et al. (1998) also found significant amounts of phthalic and terephthalic acids in emissions from motor vehicles in a roadway tunnel.
Homologous series of fatty C8–C22 straight-chain saturated monocarboxylic acids (alkanoic acids) were identified in the exhaust emissions (Fig. S5, Table S5). Among them, hexadecanoic acid was the most abundant compound in emissions from B0 and B10, followed by nonanoic and octanoic acids. In emissions from B20 and B30 blends, nonanoic acid was the dominant species, followed by hexadecenoic and octanoic acids. The highest EFs of all detected acids were registered for B20 or B30 blends, with the exception of C16 and C18 homologs, whose emissions were higher during the combustion of petrodiesel. Emissions of C8–C15 acids had very strong or strong correlations (r = 0.91–0.68) with biodiesel content (Table S5). Strong and moderate negative relationships with biodiesel content were recorded for emissions of hexadecanoic (r = 0.78) and octadecanoic (r = − 0.44) acids. Octanoic, nonanoic, decanoic, undecanoic, and dodecanoic acids showed very strong positive correlations (r = 0.97–1.0) among them, possibly indicating similar formation mechanisms of these compounds. Very strong negative correlations (r = − (0.93–0.99)) were recorded between C13 vs C16, C18 vs C9, C18 vs C10, C18 vs C12, and C19 vs C17 homologs. This suggests that the heavier acids could have decomposed, giving rise to lower molecular weight homologs. Likewise, strong positive or negative relationships were recorded for many other alkanoic acids (Table S5). According to Kawamura and Kaplan (1987), n-monocarboxylic acids can be combustion products of normal alkanes in fuels.
Regarding particle size, most of the individual EFs of alkanoic acids identified in B0 and B10 tests presented the highest value for coarse and fine and the lowest for fine fractions, respectively (Table S5). The exceptions were the hexadecanoic and octadecanoic acids, whose emissions were dominant in nanoparticles. However, in exhaust emissions from B20 and B30 blends, it was possible to observe a decrease in EFs of these acids in coarse and fine particles, while their EFs increased in ultrafine and nanoparticles for most of the homologs.
It is noteworthy that EFs of even chain fatty acids were significantly higher than emissions of odd chain fatty acids, except for C8 and C9 homologs. Cheung et al. (2010) investigated various classes of organic compounds from engine emissions fuelled with conventional diesel and 100% soybean biodiesel, reporting average EFs of C8–C28 alkanoic acids between 100 ng km−1 and 1000 μg km−1. In their study, similarly to ours, the emissions of even-chained acids were most abundant for C12–C27 homologs. Kawamura et al. (2000) documented concentrations of C1–C9 water-soluble monocarboxylic acids in gaseous and particulate phases of motor vehicle exhausts. In their study, in particulate emissions from Mercedes Benz 2200 (1971, Diesel, 2.2 l, 28000 miles), concentrations of monocarboxylic acids were dominated by odd chain homologs with an even/odd ratio of 0.68 for C5–C10 acids. Fraser et al. (1998) reported a very high emission of the order of 493.4 and 302.9 μg L−1 fuel for hexadecanoic (palmitic) and octadecanoic (stearic) acids, respectively. In the present study, EFs of C16 and C18 acids were significantly lower, ranging between 0.082–0.354 and 0.011–0.027 μg L−1 fuel, respectively.
Three nitro acids were detected in exhaust emissions (Table S5). Among them only pyroglutamic acid (5-oxo-L-proline) was present in emissions from all tested fuels, being the most abundant. Proline, glycine, and serine were found in soybean cultivars (Chavan et al. 2019; Qin et al. 2014). Also, some other water-soluble organic acids, including unsaturated and resin acids, were detected in exhaust emissions (Table S5). Generally, cis-pinonic (EFs = 14.6–64.8 ng km−1) and dehydroabietic (EFs = 10.3–75.9 ng km−1) acids were the dominant species. Pinic, citric, cis-9-octadecenoic (oleic), linoleic acid, dehydroabietic, and isopimaric acids were emitted during combustion of diesel and biodiesel blends in minor amounts.
Emissions of glycerol showed no significant changes with different types of fuel, ranging between 1.45 and 1.87 μg km−1 (all size fractions). However, its size distribution pattern changed with addition of biofuel. It was observed that in emissions from petrodiesel, the share in nanoparticles was about 17%, while the proportions in ultrafine and coarse and fine fractions were 38 and 45%, respectively. The particle size distribution of glycerol for different fuel blends was relatively homogeneous. Glycerol accounted for 28 to 37% of the total compound emissions for each size fraction. It can be present in biofuels as a contaminant (Bajpai and Tyagi 2006), which can explain its presence in exhaust emissions. Some other organic compounds, such as polyethylene glycols, urea, and fatty alcohols, were detected in exhaust emissions in insignificant amounts (Table S5). Some of these compounds are related to contaminations, lubricants, or ingredients in motor oil or catalytic additives.
Conclusions
A study on the effects of different biodiesel-diesel blends on gaseous and particulate emissions from a diesel engine with the main focus on water-soluble organic fraction was carried out. Petrodiesel and B10, B20, and B30 blends were tested in a chassis dynamometer system under transient mode. The operation conditions were kept constant between tests, the only variable being the compositional differences of the fuel. Particulate size distributions of exhaust particles were also evaluated.
The results demonstrated that biodiesel blends affected the amounts, chemical composition, and size distribution pattern of exhaust emissions. It was observed that increasing the amount of biofuel up to 30% in the blends reduced WSO emissions by 20.8% in comparison with conventional diesel. Organic acids accounted for 82–89% of WSO in emissions from all tested fuels. Dicarboxylic acids were the most abundant compound class, followed by hydroxy, aromatic, and linear alkanoic acids. Emissions of dicarboxylic and hydroxy acids showed a reduction with increasing biofuel content. Aromatic and alkanoic acids were emitted in higher amounts from combustion of B30 and B20/B30 blends, respectively. Diacids and aromatic and alkanoic acids recorded very strong or moderate correlation with biodiesel content, indicating that these compounds can possibly have the same origins during the combustion process. Significant amounts of hydroxy acids were found in WSO exhaust emissions, also showing very strong correlations with the biodiesel content. Hydroxy acids are known to be derived from biological activities or generated from photochemical oxidation of biogenic or anthropogenic precursors. Aromatic acids accounted for 23% and about 11% of identified organic acid emissions from the combustion of the B30 blend and all other fuels tested, respectively. On the contrary, the lowest content of hydroxy acids in particulate emissions was recorded for the B30 blend.
The WSO content in coarse and fine particles decreased with the increase of biofuel content in the fuel blends. Although no emission pattern was registered for ultrafine and nanoparticles, an increase in the WSO content was observed in these two fractions of finer particles from B20 and B30 blends, when comparing with petrodiesel. The biodiesel content in the fuel also affected the chemical profile of particle size-segregated WSOs. Emission factors of about 50 water-soluble organic acids from diesel engine, fuelled with different biodiesel blends, were provided. The highest EFs were found for oxalic, glycolic, benzoic, and succinic acids. Glycerol and polyethylene glycol were also emitted in noteworthy amounts. Carboxylic acids represent a significant fraction of water-soluble carbon and play an important role on the CCN activity and PM growth. Correlations between pollutants demonstrated that adding biodiesel to diesel fuel reduces the emissions of NOx, BTEX, CH4, THC, NMHC, and dicarboxylic and hydroxy acids, but increases the emissions of CO2 and alkanoic and aromatic acids. The chemical speciation of water-soluble carboxylic acids in exhaust emissions constitutes a support tool in assessing the environmental impact of engine particulate emissions and in planning air quality control strategies. The use of biodiesel as blending compound for petrodiesel will increase in the coming decades due to several environmental, economic, and social advantages. Thus, the characterisation of the chemical composition and size distributions of engine-emitted particles will be of great interest in the future.
Data availability
All data generated or analysed during this study are available from the corresponding author on reasonable request.
References
ABNT NBR 12026 (2021) Light road vehicles - determination of aldehydes and ketones in exhaust gas by liquid chromatography - DNPH method. Available in: https://www.normas.com.br/visualizar/abnt-nbr-nm/4755/abnt-nbr12026-veiculos-rodoviarios-automotores-leves-determinacao-da-emissao-de-aldeidos-e-cetonas-contidos-no-gas-de-escapamento-porcromatografia-liquida-metodo-dnph
Abu-Hamdeh NH, Alnefaie KA (2015) A comparative study of almond and palm oils as two bio-diesel fuels for diesel engine in terms of emissions and performance. Fuel 150:318–324. https://doi.org/10.1016/j.fuel.2015.02.040
Al Qubeissi M, Sazhin SS, Heikal MR (2015) Modelling of droplet heating and evaporation: an application to biodiesel, gasoline and diesel fuels. In: 8th International Conference on Thermal Engineering: Theory and Applications, Amman, Jordan. https://doi.org/10.13140/RG.2.1.4081.2561
Alptekin E, Canakci M, Ozsezen AN, Turkcan A, Sanli H (2015) Using waste animal fat based biodiesels–bioethanol–diesel fuel blends in a DI diesel engine. Fuel 157:245–254
Amaral BS, Ventura LMB, Amaral AS, Neto FRA, Gioda A (2017) Concentration profiles of regulated and unregulated pollutants emitted from the combustion of soybean biodiesel and diesel/biodiesel blend originating of a diesel cycle engine. J Braz Chem Soc 28:659–668. https://doi.org/10.21577/0103-5053.20160216
Armas O, Yehliu K, Boehman AL (2010) Effect of alternative fuels on exhaust emissions during diesel engine operation with matched combustion phasing. Fuel 89:438–456. https://doi.org/10.1016/j.fuel.2009.09.022
Ashraful AM, Masjuki HH, Kalam MA (2015) Particulate matter, carbon emissions and elemental compositions from a diesel engine exhaust fuelled with diesel-biodiesel blends. Atmos Environ 120:463–474. https://doi.org/10.1016/j.atmosenv.2015.09.028
Atabani AE, Silitonga AS, Badruddina IA, Mahlia TM, Masjuki HH, Mekhilef S (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sust Energ Rev 16(4):2070–2093. https://doi.org/10.1016/j.rser.2012.01.003
Atkinson RW, Analitis A, Samoli E, Fuller GW, Green DC, Mudway IS, Anderson HR, Kelly FJ (2016) Short-term exposure to trafficrelated air pollution and daily mortality in London UK. J Expo Sci Environ Epidemiol 26:125–132. https://doi.org/10.1038/jes.2015.65
Bajpai D, Tyagi VK (2006) Biodiesel: source, production, composition, properties and its benefits. J Oleo Sci 55:487–502. https://doi.org/10.5650/jos.55.487
Bakeas EB, Karavalakis G (2013) Regulated, carbonyl and polycyclic aromatic hydrocarbon emissions from a light-duty vehicle fueled with diesel and biodiesel blends. Environ Sci Process Impacts 15:412–422. https://doi.org/10.1039/c2em30575e
Bao L, Sakamoto K (2009) Chemical characterization of water-soluble organic acids in size-segregated particles at a suburban site in Saitama Japan. Asian J Atmospheric Environ 3(1):42–51. https://doi.org/10.5572/ajae.2009.3.1.042
Barnes I, Rudzinski KJ (2006) Environmental simulation chambers: application to atmospheric chemical processes. Springer, Polonia
Bock N, Baum MM, Anderson MB, Pesta A, Northrop WF (2017) Dicarboxylic acid emissions from aftertreatment equipped diesel engines. Environ Sci Technol 51(21):13036–13043. https://doi.org/10.1021/acs.est.7b03868
Bockey D (2019) The significance and perspective of biodiesel production – a European and global view. OCL 26:40. https://doi.org/10.1051/ocl/2019042
Bório HF, Penteado R, Daemme LC, Godoi R, Errera MR, Corrêa SM (2019) Criteria and aldehyde emissions from a diesel Euro V engine using diesel/biodiesel blends in Brazil. Environ Sci Pollut Res 26:12470–12480. https://doi.org/10.1007/s11356-019-04345-5
Borrás E, Tortajada-Genaro LA, Vázquez M, Zielinska B (2009) Polycyclic aromatic hydrocarbon exhaust emissions from different reformulated diesel fuels and engine operating conditions. Atmos Environ 43:5944–5952. https://doi.org/10.1016/j.atmosenv.2009.08.010
Casal CS, Arbilla G, Corrêa SN (2014) Alkyl polycyclic aromatic hydrocarbons emissions in diesel/biodiesel exhaust. Atmos Environ 96:107–116. https://doi.org/10.1016/j.atmosenv.2014.07.028
Chavan SV, Jadhav PV, Mane SS, Nichal SS (2019) Studies on the biochemical properties of soybean (Glycine max) genotypes in response to charcoal rot incidence. Int J Chem Stud 7(5):1109–1112
Cheung KL, Ntziachristos L, Tzamkiozis T, Schauer JJ, Samaras Z, Moore KF, Sioutas C (2010) Aerosol science and technology emissions of particulate trace elements, metals and organic species from gasoline, diesel, and biodiesel passenger vehicles and their relation to oxidative potential emissions of particulate trace elements, metals and organic aerosol. Aerosol Sci Technol 44:500–513. https://doi.org/10.1080/02786821003758294
Cheung KL, Polidori A, Ntziachristos L, Tzamkiozis T, Samaras Z, Cassee FR et al (2009) Chemical characteristics and oxidative potential of particulate matter emissions from gasoline, diesel, and biodiesel cars. Environ Sci Tech 43(16):6334–6340. https://doi.org/10.1021/es900819t
Chiang HL, Lai YM, Chang SY (2012) Pollutant constituents of exhaust emitted from light-duty diesel vehicles. Atmos Environ 47:399–406. https://doi.org/10.1016/j.atmosenv.2011.10.045
Chuepeng S, Tsolakis A, Theinnoi K, Xu HM, Wyszynski ML (2007) A study of quantitative impact on emissions of high proportion RME-based biodiesel blends SAE Technical Paper Series 2007-01-0072
Corrêa SM, Arbilla G (2006) Aromatic hydrocarbons emissions in diesel and biodiesel exhaust. Atmos Environ 40(35):6821–6826. https://doi.org/10.1016/j.atmosenv.2006.05.068
Corrêa SM, Arbilla G (2007) A two-year monitoring program of aromatic hydrocarbons in Rio de Janeiro downtown area. J Braz Chem Soc 18:539–543. https://doi.org/10.1590/S0103-50532007000300007
Correa SM, Arbilla G, da Silva CM, Martins EM, de Souza SLQ (2021) Determination of size-segregated polycyclic aromatic hydrocarbon and its nitro and alkyl analogs in emissions from diesel-biodiesel blends. Fuel 283:118912. https://doi.org/10.1016/j.atmosenv.2011.10.045
Correa SM, Arbilla G, Marques MRC, Oliveira KMPG (2012) The impact of BTEX emissions from gas stations into the atmosphere. Atmos Pollut Res 3:163–169. https://doi.org/10.5094/APR.2012.016
Da Silva CA, Conejero MA, Ribeiro ECB, Batalha MO (2019) Competitiveness analysis of “social soybeans” in biodiesel production in Brazil. Renew Energ 133:1147–1157. https://doi.org/10.1016/j.renene.2018.08.108
Daemme LC, Errera MR, Zotin MFZ, Corrêa SM, Penteado RA, Forcetto ALS (2016a) The effect of fuel sulfur content on ammonia, aldehyde and regulated emissions emitted from a Euro III motorcycle. In: SAE Technical Paper Series, São Paulo
Daemme LC, Penteado R, Corrêa SM, Zotin F, Errera MR (2016b) Emissions of criteria and non-criteria pollutants by a flex-fuel motorcycle. J Braz Chem Soc 27:2192–2202. https://doi.org/10.5935/0103-5053.20160111
Damanik N, Ong HC, Tong CW, Mahlia TMI, Silitonga AS (2018) A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ Sci Pollut Res Int 25(16):15307–15325. https://doi.org/10.1007/s11356-018-2098-8
Du Z, He K, Cheng Y, Duan F, Ma Y, Liu J, Zhang X, Zheng M, Weber R (2014) A yearlong study of water-soluble organic carbon in Beijing I: sources and its primary vs. secondary nature. Atmos Environ 92:514–521. https://doi.org/10.1016/j.atmosenv.2014.04.060
Duarte RMBO, Freire SMSC, Duarte AC (2015) Investigating the water-soluble organic functionality of urban aerosols using two-dimensional correlation of solid-state 13 C NMR and FTIR spectral data. Atmos Environ 116:245–252. https://doi.org/10.1016/j.atmosenv.2015.06.043
Duarte RMBO, Piñeiro-Iglesias M, López-Mahía P, Muniategui-Lorenzo S, Jorge Moreda-Piñeiro J, Silva AMS, Duarte C, A.C. (2019) Comparative study of atmospheric water-soluble organic aerosols composition in contrasting suburban environments in the Iberian Peninsula Coast. Sci Tot Environ 648:430–441. https://doi.org/10.1016/j.scitotenv.2018.08.171
Ema M, Naya M, Horimoto M, Kato H (2013) Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reprod Toxicol 42:1–17. https://doi.org/10.1016/j.reprotox.2013.06.074
Ferreira SL, Dos Santos AM, De Souza GR, Polito WL (2008) Analysis of the emissions of volatile organic compounds from the compression ignition engine fueled by diesel–biodiesel blend and diesel oil using gas chromatography. Energy 33(12):1801–1806. https://doi.org/10.1016/j.energy.2008.08.002
Fraser MP, Cass GR, Simoneit BRT (1998) Gas-phase and particle-phase organic compounds emitted from motor vehicle traffic in a Los Angeles roadway tunnel. Environ Sci Technol 32(14):2051–2060. https://doi.org/10.1021/es970916e
Fukagawa NK, Li M, Poynter ME, Palmer BC, Parker E, Kasumba J, Holmén BA (2013) Soy biodiesel and petrodiesel emissions differ in size, chemical composition and stimulation of inflammatory responses in cells and animals. Environ Sci Technol 47(21):12496–12504. https://doi.org/10.1021/es403146c
Garcia LFA, Corrêa SM, Penteado R et al (2013) Measurements of emissions from motorcycles and modeling its impact on air quality. J Braz Chem Soc 24:375–384. https://doi.org/10.5935/0103-5053.20130048
Ghadikolaei MA, Ka-Fu Yung KF, Cheung CS, Lau P-C (2019) Chemical properties and composition of PM emitted from a diesel engine fueled with ternary fuel (diesel-biodiesel-ethanol) in blended and fumigation modes. Fuel 251:368–382. https://doi.org/10.1016/j.fuel.2019.04.007
Ghazali WNMW, Mamat R, Masjuki HH, Najafi G (2015) Effects of biodiesel from different feedstocks on engine performance and emissions: a review. Renew Sustain Energy Rev 51:585–602. https://doi.org/10.1016/j.rser.2015.06.031
Ghio AJ, Smith CB, Madden MC (2012) Diesel exhaust particles and airway inflammation. Curr Opin Pulm Med 18:144–150. https://doi.org/10.1097/MCP.0b013e32834f0e2a
Hasan MM, Rahman MM (2017) Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: a review. Renew Sust Energ Rev 74:938–948. https://doi.org/10.1016/j.rser.2017.03.045
He C, Li J, Wang Y, Tan J, Song G, Jia D, Zhao L (2017) Size-segregated particulate matter emission characteristics of a heavy-duty diesel engine with oxygenated fuels. Appl Therm Eng 125:1173–1180. https://doi.org/10.1016/j.applthermaleng.2017.07.118
Hoekman SK (2009) Biofuels in the U.S. – challenges and opportunities. Renew Energ 34:14–22. https://doi.org/10.1016/j.renene.2008.04.030
Hoekman SK, Robbins C (2012) Review of the effects of biodiesel on NOx emissions. Fuel Process Technol 96:237–249. https://doi.org/10.1016/j.fuproc.2011.12.036
Karavalakis G, Deves G, Fontaras G, Stournas S, Samaras Z, Bakeas E (2010a) The impact of soy-based biodiesel on PAH, nitro-PAH and oxy-PAH emissions from a passenger car operated over regulated and nonregulated driving cycles. Fuel 89:3876–3883. https://doi.org/10.1016/j.fuel.2010.07.002
Karavalakis G, Stournas S, Ampatzoglou D, Bakeas E, Spanos A (2010b) Regulated and unregulated emissions of a Euro 4 SUV operated with diesel and soy-based biodiesel blends. SAE Int J Fuels Lubr 2:115–131 http://www.jstor.org/stable/26271547
Karavalakis G, Stournas S, Bakeas E (2009) Effects of diesel/biodiesel blends on regulated and unregulated pollutants from a passenger vehicle operated over the European and the Athens driving cycles. Atmos Environ 43:1745–1752. https://doi.org/10.1016/j.atmosenv.2008.12.033
Kawamura K, Kaplan IR (1987) Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air. Environ Sci Technol 21:105–110. https://doi.org/10.1021/es00155a014
Kawamura K, Steinberg S, Kaplan IR (1985) Capillary GC determination of short-chain dicarboxylic acids in rain, fog, and mist. Int J Enviro. Anal Chem 19(3):175–188. https://doi.org/10.1080/03067318508077028
Kawamura K, Steinberg S, Kaplan IR (2000) Homologous series of C1 – C10 monocarboxylic acids and C1 – C6 carbonyls in Los Angeles air and motor vehicle exhausts. Atmos Environ 34:4175–4191. https://doi.org/10.1016/S1352-2310(00)00212-0
Kawamura K, Yasui O (2005) Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere. Atmos Environ 39(10):1945–1960. https://doi.org/10.1016/j.atmosenv.2004.12.014
Kontses A, Dimaratos A, Keramidas C, Williams R, Hamje H, Ntziachristos L, Samaras Z (2019) Effects of fuel properties on particulate emissions of diesel cars equipped with diesel articulate filters. Fuel 255:115879. https://doi.org/10.1016/j.fuel.2019.115879
Kumar N, Varun CSR (2013) Performance and emission characteristics of biodiesel from different origins: a review. Renew Sustain Energ Rev 21:633–658. https://doi.org/10.1016/j.rser.2013.01.006
Kumar P, Broekhuizen K, Abbatt JPD (2003) Organic acids as cloud condensation nuclei: laboratory studies of highly soluble and insoluble species. Atmos Chem Phys 3:509–520. https://doi.org/10.5194/acp-3-509-2003
Lam MK, Lee KT, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28:500–518. https://doi.org/10.1016/j.biotechadv.2010.03.002
Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ et al (2011) Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect 119:1149–1155. https://doi.org/10.1289/ehp.1002986
Li T, Zelong WZ, Yuan B, Ye C, Lin Y, Wang S, Sha Q, Yuan Z, Zheng J, Shao M (2021) Emissions of carboxylic acids, hydrogen cyanide (HCN) and isocyanic acid (HNCO) from vehicle exhaust. Atmos Environ 247:118218. https://doi.org/10.1016/j.atmosenv.2021.118218
Li X, Zheng Y, Guan C, Li X, Zheng Y, Guan C (2018) Effect of biodiesel on PAH, OPAH, and NPAH emissions from a direct injection diesel engine. Environ Sci Pollut Res 25:34131–34138. https://doi.org/10.1007/s11356-018-3382-3
Lim C, Lee J, Hong J, Song C, Han J, Cha J-S (2014) Evaluation of regulated and unregulated emissions from a diesel powered vehicle fueled with diesel/biodiesel blends in Korea. Energy 77:533–541. https://doi.org/10.1016/j.energy.2014.09.040
Lin YC, Lee CF, Fang T (2008a) Characterization of particle size distribution from diesel engines fueled with palm-biodiesel blends and paraffinic fuel blends. Atmos Environ 42:1133–1143. https://doi.org/10.1016/j.atmosenv.2007.10.046
Lin YC, Tsai CH, Yang CR, Wu CHJ, Wu TY, Chang-Chien GP (2008b) Effects on aerosol size distribution of polyaromatic aromatic hydrocarbons from the heavy-duty diesel engine generator fueled with feedstock palm-biodiesel blends. Atmos Environ 42:6679–6688. https://doi.org/10.1016/j.atmosenv.2008.04.018
Macedo VC, Daemme LC, Penteado R et al (2017) BTEX emissions from flex fuel motorcycles. Atmos Pollut Res 8:1160–1169. https://doi.org/10.1016/j.apr.2017.05.006
Martins EM, Arbilla G, Bauerfeldt GF, De PM (2007) Atmospheric levels of aldehydes and BTEX and their relationship with vehicular fleet changes in Rio de Janeiro urban area. Chemosphere 67:2096–2103. https://doi.org/10.1016/j.chemosphere.2006.09.088
Martins EM, Borba PFDS, Dos Santos NE, Dos Reis PTB, Silveira RS, Corrêa SM (2016) The relationship between solvent use and BTEX concentrations in occupational environments. Environ Monit Assess 188:608. https://doi.org/10.1007/s10661-016-5621-8
Martins LD, da Silva Júnior CR, Solci MC, Pinto JP, Souza DZ, Vasconcellos P, Guarieiro AL, Guarieiro LL, Sousa ET, de Andrade JB (2012) Particle emission from heavy-duty engine fuelled with blended diesel and biodiesel. Environ Monit Assess 184:2663–2676. https://doi.org/10.1007/s10661-011-2142-3
Mathew GMM, Raina D, Narisetty V, Kumar V, Saran S, Pugazhendi A, Sindhu R, Pandey A (2021) Parameswaran Binod, Recent advances in biodiesel production: challenges and solutions. Sci Total Environ 794:148751. https://doi.org/10.1016/j.scitotenv.2021.148751
Mehus AA, Reed RJ, Lee VST, Littau SR, Hu C, Lutz EA, Burgess JL (2015) Comparison of acute health effects from exposures to diesel and biodiesel fuel emissions. J Occup Environ Med 57(7):705–712. https://doi.org/10.1097/JOM.0000000000000473
Meira M, Quintella CM, Ribeiro EMO, Silva HRG, Guimarães AK (2015) Overview of the challenges in the production of biodiesel. Biomass Conv Bioref 5:321–329. https://doi.org/10.1007/s13399-014-0146-2
Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T et al (2005) Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112:3930–3936. https://doi.org/10.1161/CIRCULATIONAHA.105.588962
Mkoma SL, Rocha GO, Regis ACD, Domingos JSS, Santos JVS, de Andrade SJ, Carvalho LS, de Andrade JB (2014) Major ions in PM2.5 and PM10 released from buses: the use of diesel/biodiesel fuels under real conditions. Fuel 115:109–117. https://doi.org/10.1016/j.fuel.2013.06.044
Moser BR (2009) Biodiesel production, properties, and feedstocks. In Vitro Cell Dev Biol - Plant 45:229–266. https://doi.org/10.1007/s11627-009-9204-z
Najafi G (2018) Diesel engine combustion characteristics using nanoparticles in biodiesel–diesel blends. Fuel 212:668–678. https://doi.org/10.1016/j.fuel.2017.10.001
Niu X, Pu W, Fu P, Chen Y, Xing Y, Wu D, Chen Z, Shi T, Zhou Y, Wen H, Wang X (2022) Fluorescence characteristics, absorption properties, and radiative effects of water-soluble organic carbon in seasonal snow across northeastern China. Atmos Chem Phys 22:14075–14094. https://doi.org/10.5194/acp-22-14075-2022
Palani Y, Devarajan C, Manickam D, Thanikodi S (2022) Performance and emission characteristics of biodiesel-blend in diesel engine: a review. Environ Eng Res 27(1):200338. https://doi.org/10.4491/eer.2020.338
Park S, Cho SY, Bae MS (2015) Source identification of water-soluble organic aerosols at a roadway site using a positive matrix factorization analysis. Sci Tot Environ 533:410–421. https://doi.org/10.1016/j.scitotenv.2015.07.004
Puhan S, Vedaraman N, Sankaranarayanan G, Ram BVB (2005) Performance and emission study of Mahua oil (madhuca indica oil) ethyl ester in a 4-stroke natural aspirated direct injection diesel engine. Renew Energ 30:1269–1278. https://doi.org/10.1016/j.renene.2004.09.010
Qi DH, Geng LM, Chen H, Bian YZ, Liu J, Ren XC (2009) Combustion and performance evaluation of a diesel engine fueled with biodiesel produced from soybean crude oil. Renew Energ 34(12):2706–2713. https://doi.org/10.1016/j.renene.2009.05.004
Qin P, Song W, Yang X, Sun S, Zhou X, Yang R, Li N, Hou W, Wu C, Han T, Ren G (2014) Regional distribution of protein and oil compositions of soybean cultivars in China. Crop Sci 54:1139–1146. https://doi.org/10.2135/cropsci2013.05.0314
R Core Team (2016) R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available in https://www.R-project.org/
Ramgolam K, Favez O, Cachier H, Gaudichet A, Marano F, Martinon L et al (2009) Sizepartitioning of an urban aerosol to identify particle determinants involved in the proinflammatory response induced in airway epithelial cells. Part Fibre Toxicol 6:10. https://doi.org/10.1186/1743-8977-6-10
Rocha LDS, Corrêa SM (2018) Determination of size-segregated elements in diesel-biodiesel blend exhaust emissions. Environ Sci Pollut Res 25:18121–18129. https://doi.org/10.1007/s11356-018-1980-8
Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT (1993) Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ Sci Technol 27:636–651. https://doi.org/10.1021/es00041a007
Serrano L, Lopes M, Pires N, Ribeiro I, Cascão P, Tarelho L et al (2015) Evaluation on effects of using low biodiesel blends in a EURO 5 passenger vehicle equipped with a common-rail diesel engine. Appl Energy 146:230–238. https://doi.org/10.1016/j.apenergy.2015.01.063
Souza SP, Seabra JEA, Horta Nogueira LA (2018) Feedstocks for biodiesel production: Brazilian and global perspectives. Biofuels 9(4):455–478. https://doi.org/10.1080/17597269.2017.1278931
Souza SR, Vasconcellos PC, Carvalho LRF (1999) Low molecular weight carboxylic acids in an urban atmosphere: Winter measurements in São Paulo City Brazil. Atmos Environ 33(16):2563–2574. https://doi.org/10.1016/S1352-2310(98)00383-5
Tang S, Zhou X, Zhang J et al (2020) Characteristics of water-soluble organic acids in PM2.5 during haze and Chinese Spring Festival in winter of Jinan, China: concentrations, formations, and source apportionments. Environ Sci Pollut Res 27:12122–12137. https://doi.org/10.1007/s11356-020-07714-7
Tsai JH, Chen SJ, Huang KL, Lin YC, Lee WJ, Lin CC, Lin WY (2010) PM, carbon, and PAH emissions from a diesel generator fuelled with soy-biodiesel blends. J Hazard Mater 179:237–243. https://doi.org/10.1016/j.jhazmat.2010.02.085
Verma P, Stevanovic S, Zare A, Dwivedi G, Chu Van T, Davidson M, Rainey T, Brown RJ, Ristovski ZD (2019) An overview of the influence of biodiesel, alcohols, and various oxygenated additives on the particulate matter emissions from diesel engines. Energies 12(10):1987. https://doi.org/10.3390/en12101987
Wang B, Or WH, Lee SC, Leung YC, Organ B, Ho KF (2021) Characteristics of particle emissions from light duty diesel vehicle fueled with ultralow sulphur diesel and biodiesel blend. Atmos Pollut Res 12:101169. https://doi.org/10.1016/j.apr.2021.101169
Wang Y, Liu H, Lee CFF (2016) Particulate matter emission characteristics of diesel engines with biodiesel or biodiesel blending: a review. Renew Sust Energ Rev 64:569–581. https://doi.org/10.1016/j.rser.2016.06.062
Xu Y, Nadykto AB, Yu F, Herb J, Wang W (2010) Interaction between common organic acids and trace nucleation species in the Earth’s atmosphere. J Phys Chem A 114(1):387–396. https://doi.org/10.1021/jp9068575
Yu JZ, Yang H, Zhang HY, Lau AKH (2004) Size distributions of watersoluble organic carbon in ambient aerosols and its size-resolved thermal characteristics. Atmos Environ 38:1061–1071. https://doi.org/10.1016/j.atmosenv.2003.10.049
Yu S (2000) Role of organic acids (formic, acetic, pyruvic and oxalic) in the formation of cloud condensation nuclei (CCN): a review. Atmos Res 53(4):185–217. https://doi.org/10.1016/S0169-8095(00)00037-5
Zhang R, Suh I, Zhao J, Zhang D, Fortner EC, Tie X, Molina LT, Molina MJ (2004) Atmospheric new particle formation enhanced by organic acids. Science 304(5676):1487–1490. https://doi.org/10.1126/science.1095139
Zhang X, Gao G, Li L, Wu Z, Hu Z, Deng J (2008) Characteristics of combustion and emissions in a DI engine fueled with biodiesel blends from soybean oil. SAE International. SAE Technical Paper Series
Živković S, Veljković M (2018) Environmental impacts the of production and use of biodiesel. Environ Sci Pollut Res 25:191–199. https://doi.org/10.1007/s11356-017-0649-z
Funding
Open access funding provided by FCT|FCCN (b-on). The sampling campaign and analytical work were supported by the project “SOPRO: Chemical and toxicological SOurce PROfiling of particulate matter in urban air”, POCI-01-0145-FEDER-029574, funded by FEDER, through COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES. Margarita Evtyugina benefited from a grant SFRH/BPD/123176/2016 given by the Portuguese Foundation for Science and Technology (FCT). This work was also supported by CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020) from FCT/MCTES through national funds, and co-funding by FEDER, within the PT2020 Partnership Agreement and Compete 2020.
Author information
Authors and Affiliations
Contributions
Margarita Evtyugina: investigation, writing - original draft; Catia Gonçalves: investigation, writing - review and editing; Célia Alves: conceptualisation, supervision, funding acquisition, writing - review and editing; Sergio M. Corrêa: conceptualisation, supervision, funding acquisition, writing - review and editing; Luiz Carlos Daemme: investigation, writing - review and editing; Renato de Arruda Penteado Neto: investigation, writing - review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors voluntarily agreed to participate in this study.
Consent for publication
All authors have agreed to publish.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
The online version contains supplementary material available at XXXX (DOCX 529 KB).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evtyugina, M.G., Gonçalves, C., Alves, C. et al. Exhaust emissions of gaseous and particle size-segregated water-soluble organic compounds from diesel-biodiesel blends. Environ Sci Pollut Res 30, 63738–63753 (2023). https://doi.org/10.1007/s11356-023-26819-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26819-3