Abstract
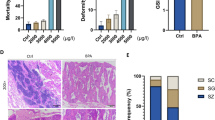
Bisphenol A (BPA) is one of the most common environmental endocrine disruptor chemicals (EDCs) and exhibits reproductive, cardiovascular, immune, and neurodevelopmental toxic effects. The development of the offspring was examined in the present investigation to determine the cross-generational effects of long-term exposure of parental zebrafish to environmental concentrations of BPA (15 and 225 µg/L). Parents were exposed to BPA for 120 days, and their offspring were evaluated at 7 days after fertilization in BPA-free water. The offspring exhibited higher mortality, deformity, and heart rates, and showed significant fat accumulation in abdominal region. RNA-Seq data showed that more lipid metabolism-related KEGG pathways, such as the PPAR signaling pathway, adipocytokine signaling pathway, and ether lipid metabolism pathway were enriched in the 225 µg/L BPA-treated offspring compared to 15 µg/L BPA-treated offspring, indicating greater effects of high dose BPA on offspring lipid metabolism. Lipid metabolism-related genes implied that BPA is responsible for disrupting lipid metabolic processes in the offspring through increased lipid production, abnormal transport, and disruption of lipid catabolism. The present study will be helpful for further evaluation of the reproductive toxicity of environmental BPA to organisms and the subsequent parent-mediated intergenerational toxicity.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Acconcia F, Pallottini V, Marino M (2015) Molecular mechanisms of action of BPA. Dose-Response 13:4
Axelstad M, Hass U, Scholze M, Christiansen S, Boberg J (2018) EDC IMPACT: reduced sperm counts in rats exposed to human relevant mixtures of endocrine disrupters. Endocr Connect 7:139–148
Birceanu O, Servos MR, Vijayan MM (2015) Bisphenol A accumulation in eggs disrupts the endocrine regulation of growth in rainbow trout larvae. Aquat Toxicol 161:51–60
Bonen A, Chabowski A, Luiken JJF, Glatz JFC (2007) Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology 22(1):15–29
Cabana H, Jones JP, Agathos SN (2010) Elimination of endocrine disrupting chemicals using white rot fungi and their lignin modifying enzymes: a review. Eng Life Sci 7(5):429–456
Chmurzyńska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47(1):39–48
Ghassabian A, Trasande L (2018) Disruption in thyroid signaling pathway: a mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front Endocrinol 9(5):204
Gimeno P, Spinau C, Lassu N, Maggio AF, Brenier C, Lempereur L (2015) Identification and quantification of bisphenol A and bisphenol B in polyvinylchloride and polycarbonate medical devices by gas chromatography with mass spectrometry. J Sep Sci 38(21):3727–3734
Haunerland NH, Spener F (2004) Fatty acid-binding proteins – insights from genetic manipulations. Prog Lipid Res 43(4):328–349
Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Volkel W, Wollin KM, Gundert-Remy U (2011) Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol 41(4):263–291
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Huang RP, Liu ZH, Yin H, Dang ZP, Wu X, Zhu NW, Lin Z (2018) Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: a thorough literature review. Sci Total Environ 626:971–981
Jakobsson A, Westerberg R (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45(3):237–249
Klosin A, Lehner B (2016) Mechanisms, timescales and principles of trans-generational epigenetic inheritance in animals. Curr Opin Genet Dev 36:41–49
Lee S, Liao C, Song GJ, Kongtae R, Kurunthachalam K, Hyo-Bang M (2015) Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 119:1000–1006
Lehmler HJ, Liu BY, Gadogbe M, Bao W (2018) Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: the national health and nutrition examination survey 2013–2014. ACS Omega 3(6):6523–6532
Liu J, Zhu XS (2016) Ionic liquid-immobilized expanded perlite solid-phase extraction for separation/analysis of bisphenol A in food packaging material. Food Anal Method 9(3):605–613
Livak KJ, Schmittgen TD (2002) Analysis of relative gene expression data using real-time quantitative pcr. Methods 25:402–408
Lombo M, Fernandez-Diez C, Gonzalez-Rojo S, Navarro C, Robles V, Herraez MP (2015) Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ Pollut 206:667–678
Lombo M, Gonzalez-Rojo S, Fernandez-Diez C, Herraez MP (2019) Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ Pollut 246:1008–1019
Martínez R, Esteve-Codina A, Herrero-Nogareda L, Ortiz-Villanueva E, Barata C, Tauler R, Raldua D, Pina B, Navarro-Martin L (2018) Dose-dependent transcriptomic responses of zebrafish eleutheroembryos to bisphenol A. Environ Pollut 243:988–997
Martínez R, Herrero-Nogareda L, Van Antro M, Campos MP, Casado M, Barata C, Pina B, Navarro-Martín L (2019) Morphometric signatures of exposure to endocrine disrupting chemicals in zebrafish eleutheroembryos. Aquat Toxicol 214:105232
McElligott MB, O’Malley DM (2005) Prey tracking by larval zebrafish: axial kinematics and visual control. Brain Behav Evolut 66(3):177–196
Melo C, Gallardo D, Quintanilla R, Zidi A, Castello A, Diaz I, Amills M, Pena RN (2013) An association analysis between polymorphisms of the pig solute carrier family 27A (SLC27A), member 1 and 4 genes and serum and muscle lipid traits. Livest Sci 152:143–146
Mi KH, Chen X, Lu KY, Zhu YJ, Zhang M, Yang H, Wei WZ, Zhang YY (2021) Bisphenol A induces hepatic triglyceride level in adult male rare minnow Gobiocypris rarus. Ecotox Environ Safe 213(8):112050
Migliarini B, Piccinetti CC, Martella A, Maradonna F, Gioacchini G, Carnevali O (2012) Perspectives on endocrine disruptor effects on metabolic sensors. Gen Comp Endocr 175(1):214
Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y (2011) The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PLoS ONE 6(10):e25865
Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H (2010) Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Persp 118(9):1196–1203
Ozhan K, Kocaman E (2019) Temporal and spatial distributions of bisphenol A in marine and freshwaters in Turkey. Arch Environ Con Tox 76:246–254
Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118(3):829–838
Qin JY, Ru SG, Wang WW, Hao LP, Wei SH, Zhang J, Xiong JQ, Wang J, Zhang XN (2021) Unraveling the mechanism of long-term bisphenol S disrupted ovarian lipids metabolism, oocytes maturation, and offspring development of zebrafish. Chemosphere 277:130304
Quinlivan VH, Farber SA (2010) Lipid uptake, metabolism, and transport in the larval zebrafish. Front Endocrinol 8:319
Rezaee M, Yamini Y, Shariati S, Esrafili A, Shamsipur M (2009) Dispersive liquid–liquid microextraction combined with high-performance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples. J Chromatogr A 1216(9):1511–1514
Richieri G, Ogata RT, Zimmerman AW, Veerkamp JH, Kleinfeld AM (2000) Fatty acid binding proteins from different tissues show distinct patterns of fatty acid. Biochemistry 39(24):7197–7204
Staples C, van der Hoeven N, Clark K, Mihaich E, Woelz J, Hentges S (2018) Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 201:448–458
Wang B, Huang B, Jin W, Zhao S, Li F, Hu P, Pan X (2013) Occurrence, distribution, and sources of six phenolic endocrine disrupting chemicals in the 22 river estuaries around Dianchi Lake in China. Environ Sci Pollut R 20:3185–3194
Wang WW, Zhang XN, Qin JY, Wei PH, Jia Y, Wang J, Ru SG (2019) Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring. Chemosphere 221:500–510
Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ (2012) Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology 153(8):3828–3838
Wu NHC, Seebacher F (2020) Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: a meta-analysis. Global Change Biol 26:3821–3833
Yang H, Xiong HR, Mi KH, Xue W, Zhang YY (2020) Toxicity comparison of nano-sized and micron-sized microplastics to goldfish Carassius auratus larvae. J Hazard Mater 388:122058
Zhang YY, Wang LF, Zhuang H, Li XX, Gao XJ, An ZH, Liu XD, Yang H, Wei WZ, Zhang XJ (2019) Excessive use of enrofloxacin leads to growth inhibition of juvenile giant freshwater prawn Macrobrachium rosenbergii. Ecotox Environ Safe 169:344–352
Zhang YY, Li TJ, Pan CY, Khan IA, Chen Z, Yue YH, Yang M (2022) Intergenerational toxic effects of parental exposure to bisphenol AF on offspring and epigenetic modulations in zebrafish. Sci Total Environ 823:153714
Zhu Z, Wang J, Cao Q, Liu S, Wei W, Yang H, Zhang Y (2022) Long-term BPA exposure leads to bone malformation and abnormal expression of MAPK/Wnt/FoxO signaling pathway genes in zebrafish offspring. Ecotox Environ Safe 252:109239
Zulkifli S, Rahman AA, Kadir SHSA, Nor NSM (2021) Bisphenol A and its effects on the systemic organs of children. Eur J Pediatr 180:3111–3127
Funding
Financial support for this study was provided by Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3557), National Natural Science Foundation of China (31800435), Postdoctoral Research Foundation of China (2020M681743), and Postdoctoral Science Foundation of Jiangsu Province (2020Z104).
Author information
Authors and Affiliations
Contributions
Zhu Zhu: methodology, validation, formal analysis, investigation, and data curation. Ziying Wang: methodology, formal analysis, investigation, and writing. Jiayu Wang: methodology and investigation. Qingsheng Cao: investigation and writing — review and editing. Hui Yang: methodology and investigation. Yingying Zhang: validation, formal analysis, Investigation, and data curation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal ethics committee of Yangzhou University approved the experimental protocol used in this study.
Consent for publication
All the authors agreed to the publication, and current article does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Z., Wang, Z., Wang, J. et al. Transcriptomic analysis of lipid metabolism in zebrafish offspring of parental long-term exposure to bisphenol A. Environ Sci Pollut Res 30, 51654–51664 (2023). https://doi.org/10.1007/s11356-023-25844-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25844-6