Abstract
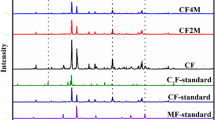
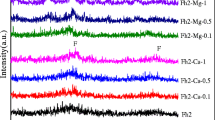
Metastable ferrihydrite is omnipresent in environments and can influence the fate of Pb(II) during ferrihydrite transformation. Ferrihydrite is rarely pure and often coexists with impurities, which may influence the mineralogical changes of ferrihydrite and Pb(II) behavior. In this work, we investigated the effect of malic acid or phosphate on Pb(II)-ferrihydrite coprecipitates (Fh-Pb) transformation and the subsequent fate of Pb(II) during the 10-day aging of Fh-Pb. Results showed that both malic acid and phosphate retarded Fh-Pb transformation and prevented the release of Pb(II) from Fh-Pb back into solutions. Pb(II) was beneficial to goethite formation by inhibiting hematite formation while both malic acid and phosphate inhibited goethite formation since they could act as templates of nucleation. Besides, malic acid and phosphate improved the proportion of non-extracted Pb(II) during Fh-Pb transformation, indicating that Pb(II) was incorporated into secondary minerals. Pb(II) could not replace Fe(III) within the crystal lattice due to its large radius but was occluded into pores and defect structures within the secondary mineral lattices. This work can advance our understanding of the influences of malic acid and phosphate on Pb(II) immobility during Fh-Pb aging.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article [and its Supplementary Information files].
References
Ahmad A, Rutten S, Eikelboom M, de Waal L, Bruning H, Bhattacharya P, van der Wal A (2020) Impact of phosphate, silicate and natural organic matter on the size of Fe(III) precipitates and arsenate co-precipitation efficiency in calcium containing water. Sep Purif Technol 235:116117
Amini M, Antelo J, Fiol S, Rahnemaie R (2020) Modeling the effects of humic acid and anoxic condition on phosphate adsorption onto goethite. Chemosphere 253:126691
Balint R, Celi L, Barberis E, Prati M, Martin M (2020) Organic phosphorus affects the retention of arsenite and arsenate by goethite. J Environ Qual 49:1655–1666
Cornell RM, Schwertmann U (1979) Influence of organic anions on the crystallization of ferrihydrite. Clay Clay Min 27:402–410
Dai C, Zhao J, Giammar DE, Pasteris JD, Zuo X, Hu Y (2018) Heterogeneous lead phosphate nucleation at organic-water interfaces: implications for lead immobilization. ACS Earth Space Chem 2:869–877
Das S, Hendry MJ, Essilfie-Dughan J (2011) Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ Sci Technol 45:268–275
Ding Y, Song Z, Feng R, Guo J (2014) Interaction of organic acids and pH on multi-heavy metal extraction from alkaline and acid mine soils. Int J Environ Sci Technol 11:33–42
Engel M, Pacheco JSL, Noël V, Boye K, Fendorf S (2021) Organic compounds alter the preference and rates of heavy metal adsorption on ferrihydrite. Sci Total Environ 750:141485
Ford RG, Bertsch PM, Farley KJ (1997) Changes in transition and heavy metal partitioning during hydrous iron oxide aging. Environ Sci Technol 31:2028–2033
Ford RG, Kemner KM, Bertsch PM (1999) Influence of sorbate-sorbent interactions on the crystallization kinetics of nickel- and lead-ferrihydrite coprecipitates. Geochim Cosmochim Acta 63:39–48
Francisco PCM, Sato T, Otake T, Kasama T, Suzuki S, Shiwaku H, Yaita T (2018) Mechanisms of Se(IV) co-precipitation with ferrihydrite at acidic and alkaline conditions and its behavior during aging. Environ Sci Technol 52:4817–4826
Gálvez N, Barrón V, Torrent J (1999) Effect of phosphate on the crystallization of hematite, goethite, and lepidocrocite from ferrihydrite. Clay Clay Min 47:304–311
Garau G, Lauro GP, Diquattro S, Garau M, Castaldi P (2019) Sb(V) adsorption and desorption onto ferrihydrite: influence of pH and competing organic and inorganic anions. Environ Sci Pollut Res 26:27268–27280
Ilton ES, Pacheco JSL, Bargar JR, Shi Z, Liu J, Kovarik L, Engelhard MH, Felmy AR (2012) Reduction of U(VI) incorporated in the structure of hematite. Environ Sci Technol 46:9428–9436
Johnson SE, Loeppert RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234
Kleine BI, Stefánsson A, Kjartansdóttir R, Prause S, Weisenberger TB, Reynolds HI, Sveinbjörnsdóttir ÁE, Jackson MD, Gudmundsson MT (2020) The surtsey volcano geothermal system: an analogue for seawater-oceanic crust interaction with implications for the elemental budget of the oceanic crust. Chem Geol 550:119702
Kukkadapu RK, Zachara JM, Fredrickson JK, Smith SC, Dohnalkova AC, Russell CK (2003) Transformation of 2-line ferrihydrite to 6-line ferrihydrite under oxic and anoxic conditions. Am Mineral 88:1903–1914
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Li Z, Shakiba S, Deng N, Chen J, Louie SM, Hu Y (2020) Natural organic matter (NOM) imparts molecular-weight-dependent steric stabilization or electrostatic destabilization to ferrihydrite nanoparticles. Environ Sci Technol 54:6761–6770
Liang Y, Yu D, Jin J, Xiong J, Hou J, Wang M, Tan W (2021) Microstructure of Al-substituted goethite and its adsorption performance for Pb(II) and As(V). Sci Total Environ 790:148202
Liu J, Louie SM, Pham C, Dai C, Liang D, Hu Y (2019) Aggregation of ferrihydrite nanoparticles: effects of pH, electrolytes, and organics. Environ Res 172:552–560
Liu J, Zhu R, Ma L, Fu H, Lin X, Parker SC, Molinari M (2021) Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: a comparative study on ferrihydrite, goethite, and hematite. Geoderma 383:114799
Lopes G, Li W, Siebecker MG, Sparks DL, Guilherme LRG (2021) Combining zinc desorption with EXAFS speciation analysis to understand Zn mobility in mining and smelting affected soils in Minas Gerais, Brazil. Sci Total Environ 754:142450
Lu Y, Hu S, Liang Z, Zhu M, Wang Z, Wang X, Liang Y, Dang Z, Shi Z (2020a) Incorporation of Pb(II) into hematite during ferrihydrite transformation. Environ Sci Nano 7:829–841
Lu Y, Hu S, Liu F, Liang Y, Shi Z (2020b) Effects of humic acid and fulvic acid on the sequestration of copper and carbon during the iron oxide transformation. Chem Eng J 383:123194
Martínez CE, Sauvé S, Jacobson A, Mcbride MB (1999) Thermally induced release of adsorbed Pb upon aging ferrihydrite and soil oxides. Environ Sci Technol 33:2016–2020
Mertz S, Le Forestier L, Bataillard P, Devau N (2021) Leaching of trace metals (Pb) from contaminated tailings amended with iron oxides and manure: new insight from a modelling approach. Chem Geol 579:120356
Mikutta C (2011) X-ray absorption spectroscopy study on the effect of hydroxybenzoic acids on the formation and structure of ferrihydrite. Geochim Cosmochim Acta 75:5122–5139
Mikutta C, Frommer J, Voegelin A, Kaegi R, Kretzschmar R (2010) Effect of citrate on the local Fe coordination in ferrihydrite, arsenate binding, and ternary arsenate complex formation. Geochim Cosmochim Acta 74:5574–5592
Neyens E, Baeyens J (2003) A review of thermal sludge pre-treatment processes to improve dewaterability. J Hazard Mater 98:51–67
Noerpel MR, Lee SS, Lenhart JJ (2016) X-ray analyses of lead adsorption on the (001), (110), and (012) hematite surfaces. Environ Sci Technol 50:12283–12291
Nordstrom DK, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. Appl Geochem 57:3–16
Perez JPH, Tobler DJ, Freeman HM, Brown AP, Hondow NS, van Genuchten CM, Benning LG (2021) Arsenic species delay structural ordering during green rust sulfate crystallization from ferrihydrite. Environ Sci Nano 8:2950–2963
Salama W, El Aref M, Gaupp R (2015) Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1816–1826
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory: preparation and characterization. VCH Verlagsgesellschaft, Weinheim/VCH Publishers, New York. https://doi.org/10.1002/9783527613229
Senn AC, Kaegi R, Hug SJ, Hering JG, Mangold S, Voegelin A (2017) Effect of aging on the structure and phosphate retention of Fe(III)-precipitates formed by Fe(II) oxidation in water. Geochim Cosmochim Acta 202:341–360
Sileo EE, Solis PS, Paiva-Santos CO (2003) Structural study of a series of synthetic goethites obtained in aqueous solutions containing cadmium(II) ions. Powder Diffr 18:50–55
Suber L, Foglia S, Fiorani D, Romero H, Montone A, Roig A, Casas L (2004) Synthesis, morphological-structural characterization and magnetic properties of amorphous iron (III)-oxyhydroxy-phosphate nanoparticles. J Solid State Chem 177:2440–2448
Tadic M, Trpkov D, Kopanja L, Vojnovic S, Panjan M (2019) Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J Alloys Compd 792:599–609
Tiberg C, Sjöstedt C, Persson I, Gustafsson JP (2013) Phosphate effects on copper(II) and lead(II) sorption to ferrihydrite. Geochim Cosmochim Acta 120:140–157
Tiberg C, Sjöstedt C, Eriksson AK, Klysubun W, Gustafsson JP (2020) Phosphate competition with arsenate on poorly crystalline iron and aluminum (hydr)oxide mixtures. Chemosphere 255:126937
Trivedi P, Dyer JA, Sparks DL (2003) Lead sorption onto ferrihydrite. 1. A macroscopic and spectroscopic assessment. Environ Sci Technol 37:908–914
Vithana CL, Johnston SG, Dawson N (2018) Divergent repartitioning of copper, antimony and phosphorus following thermal transformation of schwertmannite and ferrihydrite. Chem Geol 483:530–543
Vu HP, Shaw S, Benning LG (2008) Transformation of ferrihydrite to hematite: an in situ investigation on the kinetics and mechanisms. Mineral Mag 72:217–220
Vu HP, Shaw S, Brinza L, Benning LG (2013) Partitioning of Pb(II) during goethite and hematite crystallization: implications for Pb transport in natural systems. Appl Geochem 39:119–128
Wei W, Cui J, Wei Z (2014) Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 105:14–23
Yang J, Liu J, Hu Y, Rumpel C, Bolan N, Sparks D (2017) Molecular-level understanding of malic acid retention mechanisms in ternary kaolinite-Fe(III)-malic acid systems: the importance of Fe speciation. Chem Geol 464:69–75
Zhang Y, Li H, Jiang Q, Jiang S, Wang Y, Wang L (2021) One-pot synthesis of a novel P-doped ferrihydrite nanoparticles for efficient removal of Pb(II) from aqueous solutions: performance and mechanism. J Environ Chem Eng 9:105721
Zhu J, Pigna M, Cozzolino V, Caporale AG, Violante A (2011) Sorption of arsenite and arsenate on ferrihydrite: effect of organic and inorganic ligands. J Hazard Mater 189:564–571
Zhu J, Fu Q, Qiu G, Liu Y, Hu H, Huang Q, Violante A (2019) Influence of low molecular weight anionic ligands on the sorption of heavy metals by soil constituents: a review. Environ Chem Lett 17:1271–1280
Funding
This research was supported by the National Natural Science Foundation of China (No. 51978174), and the Natural Science Foundation of Guangdong Province (No. 2018A030313099).
Author information
Authors and Affiliations
Contributions
Jinlong Peng: methodology, investigation, data curation, writing—original draft. Fenglian Fu: conceptualization, supervision, writing—review and editing, funding acquisition. Lin Zhang: writing—review and editing. Bing Tang: writing—review and editing. Xiangdan Zhang: writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This part is not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, J., Fu, F., Zhang, L. et al. Enhanced immobility of Pb(II) during ferrihydrite-Pb(II) coprecipitates aging impacted by malic acid or phosphate. Environ Sci Pollut Res 30, 45899–45909 (2023). https://doi.org/10.1007/s11356-023-25541-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25541-4