Abstract
There is a dearth of data on rare earth elements (REE), yttrium and scandium in foods which extends also to baseline datasets for edible wild mushrooms, though this has started to change in the last decade. Concentrations and shale normalized patterns of REE and Y (REY) were studied by using inductively coupled plasma–quadrupole mass spectrometer in 22 pools (2235 specimens) of Cantharellus cibarius (Golden Chanterelle) collected in Poland and also a pool of C. minor (Small Chanterelle) (153 specimens) from Yunnan (Chinese Province). The total REY plus Sc varied in C. cibarius from 10 to 593 µg kg−1 dw whereas that for the Yunnan’s C. minor was 2072 µg kg−1 dw. C. minor from Yunnan has higher REY and Sc compared to the C. cibarius. Sc concentrations in twenty C. cibarius pools were below 1 µg kg−1 dw, but 17 and 27 µg kg−1 dw were detected at the other two sites and 66 µg kg−1 dw was detected in C. minor. The median Y content of C. cibarius and C. minor was 22 µg kg−1 dw and 200 µg kg−1 dw. The difference in REY and Sc concentrations and shale normalized patterns between mushrooms from Poland and Yunnan seems to reflect the regional difference in concentration and composition of these elements in the soil bedrock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REE) are elements from the group of lanthanides (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), including scandium (Sc) and yttrium (Y) that are precious metals and are increasingly used in the manufacture of electrical and electronic devices and other developing technologies for industrial, home, and personal devices (Migaszewski and Gałuszka 2015; Migaszewski et al. 2016; Balaram 2019). Estimated mean concentration of REE in the earth’s crust ranges from about 130 to 240 mg kg−1, which is in fact much higher than other commonly mined elements and much higher than their corresponding abundances in chondrites (Zepf 2013; Balaram 2019). REE and other elements have become an emerging field of investigation in environmental and food sciences (Aruguete et al. 1998; Migaszewski and Gałuszka 2015; Pagano et al. 2019; Zabowski et al. 1990).
The increasing use of the REE as a whole or in various individual REE applications in the economy can be considered possible sources of REE in forest soils and so also of their accumulation in wild mushrooms. Coal fly ash contains a certain amount of REE (Franus et al. 2015) and as an anthropogenic waste it can be considered a diffusive source of REE deposition in the ground, although it may generally be a poor source. Sewage sludge from wastewater treatment plants can contain REE and especially if such a facility receives input from specific industrial sources that could contain REE (Kaegi et al. 2021). Treated municipal sewage sludge can possibly be used in forested lands (Zabowski et al. 1990) and/or in agricultural soils (under certain circumstances and regulations as seen in Poland) and this could be a source of REE for forest and field mushrooms. Also, phosphorous fertilizers contain some quantities of REE (Volk et al. 1990). Nevertheless, there is lack of data on the effect of using sewage sludge or phosphorous fertilizers (containing REE) in agriculture and the occurrence of REE in mushrooms grown in treated forests or agricultural soils or from cultivars.
Knowledge of the presence of REE in food, including edible mushrooms (fungi), is limited due to trace to ultra-trace levels, lack of robust analytical methods in the past, reliability of published data and lack of information on the risk of REE as potential food contaminants (Balaram 2019; Borovička et al. 2011; Falandysz et al. 2001; Falandysz 2023a, b, c; Grawunder and Gube 2018; Stijve et al. 2004; Zocher et al. 2018). The first data on the occurrence of 14 REE in mushrooms, including species: Armillaria solidipes (A. ostoyae, Armillaria Root Rot), Boletus edulis (King Bolete), Laccaria amethystina (Amethyst Deceiver), Suillus bovinus (Jersey Cow Mushroom or Bovine Bolete), Suillus luteus (Slippery Jack), Tricholoma equestre (T. flavovirens, Yellow Knight, Man on Horseback or Saddle-shaped Tricholoma), were published in 2001 (Falandysz et al. 2001). As determined by double focusing sector field inductively coupled plasma mass spectrometry, the individual REE were found to be present in these mushrooms in the range from 0.60 µg kg−1 dry weight (dw) for Eu to 400 µg kg−1 dw for Ce while A. solidipes showed relatively higher concentrations of Ce, Nd and La than the other mushroom species (Falandysz et al. 2001). Scandium (Sc) and yttrium (Y) are also considered by some authors along with the REE, but their occurrence in mushrooms was not discussed together with the other lanthanides in earlier studies (Borovička et al. 2011; Falandysz et al. 2001; Grawunder and Gube 2018; Stijve et al. 2002, 2004; Zocher et al. 2018). Soil REE, Y, and Sc levels are a driver of their accumulation in vegetation, including mushrooms (Dołęgowska and Migaszewski 2013; Ichihashi et al. 1992; Markert and Li 1991; Mędyk and Falandysz, 2022; Zocher et al. 2018).
The mycelium absorbs various elements, including REE and toxic elements (e.g., As, Cd, and Hg) from soil and other substrates (with varying absorption rates) and then translocates these to fruiting bodies or the sclerotia (Andersson et al. 2018; Saba et al. 2020; Tyler 1982; Yoshida and Muramatsu 1997; Zhang et al. 2022). Bioconcentration factor (BCF) is a parameter used to quantify the potential of organism to bioconcentrate mineral constituents in abiotic relationships, e.g., macromycete–soil. The BCF is expressed as the quotient of the concentration of an element in the fruiting body to the level in the substrate (e.g., soil) on dry to dry weight basis. BCF values of La and Ce in mushrooms such as Amanita pantherina (common name Panthercap mushroom or False Blusher), Lactarius hatsudake (Hatsu Take), Russula mariae (Purple-bloom), Suillus granulatus (Dotted-stem Bolete, Granulated Bolete or Ringless Slippery Jack) and T. equestre have been reported to be well below 1, i.e., they showed on bio-exclusion of REE by fungi, with reported quotients ranging from 0.003 to 0.027 for La and from 0.0003 to 0.025 for Ce (Tyler 1982; Yoshida and Muramatsu 1997).
Knowledge of the occurrence and possible role or not of REE in the physiology of macromycetes is negligible so far. Nevertheless, macromycetes accumulate some amounts of REE in fruiting bodies. Generating reliable baseline data sets on the mineral constituent concentration of raw mushrooms from wild biodiversity is the first step in assessing possible dietary intakes and likely health effects. However, other factors to consider when assessing actual consumption include the effect of culinary processing and preservation, and the accessibility/bioavailability of the minerals after ingestion — the release of minerals from the mushroom meal, and intestinal absorption and bioactivity. Venturella et al. (2014) investigated 13 lanthanides and reported the values of Eu Tb, Dy, Ho, Er Tm, Yb, and Lu, below the detection limit, i.e., from 1 to 2 µg kg−1 dw. The study also showed no accumulation factor for Eu in Suillellus queletii (Deceiving Bolete) (earlier name Boletus queletii) and Leccinellum lepidum (Neat Bolete) as well as Er in B. queletii and L. lepidum, and Yb in B. queletii and L. lepidum. It was also observed that the f-block lanthanides (Ce, Pr, Nd, Sm, and Gd) do not have a bioconcentration factor, except for Sm, which has a value that is below the detection limit for Rubroboletus satanas (Devil’s Bolete) (earlier name Boletus satanas) (Venturella et al. 2014).
Cantharellus cibarius (Golden Chanterelle, Common Chanterelle, Girolle) is a popular species in Europe and fresh mushrooms a very popular seasonal wild food product there as well as in the Northern Hemisphere. That species has been widely characterised for its macro- and micro-nutrient levels (e.g., K, P, S, Mg, Mn, Na, Ca, Cu, Zn, Co and Se) and radiotoxic 137Cs (Bakaytis et al. 2021; Drewnowska and Falandysz 2015; Drewnowska et al. 2017; Falandysz and Drewnowska 2015; Falandysz et al. 2016; Mędyk et al. 2017; Mirończuk-Chodakowska et al. 2019; Mleczek et al. 2013). Cantharellus minor is a mycorrhizal, edible species, much smaller than the average C. cibarius, but has more slender proportions (Kuo 2006). No data on mineral constituents in C. minor could be found in the available scientific literature.
There is a dearth of data on the REE content of mushrooms, especially where credible, validated analytical methods were used. The ambiguity of some published REE datasets for mushrooms has been raised clearly (sample cross contamination with soil/sand particles, lack of method sensitivity and resolution, poor limit of detection, poor choice of instrument, spectral interferences) (Borovička et al. 2011; Stijve et al. 2004; Zocher et al. 2018). This derives more from the very low concentrations at which these elements occur compared to some reported values as well as the analytical chemistry approach adopted (methodology, materials and instrumentation or external contamination) (Borovička et al. 2011; Stijve et al. 2004; Zocher et al. 2018). However, the baseline rules when determining REE in mushrooms is the adoption of adequate sampling in terms of the quantity of the fruiting bodies examined (individual sample or preferably as composite samples), sample preparation approach (clean-up from soil particles and risk of particle incrustation within a fruiting body) and the proper choice of analytical method/instrumentation, including use of argon plasma gas in mass spectrometry and the elimination of interferences (Balaram 2019; Bau et al. 2018; Prohaska et al. 1999; Stijve et al. 2004; Zawisza et al. 2011). Golden chanterelles are a very popular seasonal, organic food product across Europe, and this study aimed to characterize the presence of REE in a relatively large sample of this mushroom from Poland. A pooled sample of C. minor collected at Yunnan is also included.
Materials and methods
Mushrooms — collection and preparation
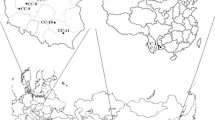
Specimens (n = 2235) of C. cibarius were pooled into 22 composites corresponding to 22 locations across Poland and collected between July–September of 1998–2008. Poland has a moderate and changing climate and landscape flat with Świetokrzyskie, Sudety, Tatra, and Carpathian Mts in the south, and agricultural and forested land dominate. Samples of C. minor (153 specimens pooled into 1 composite sample) were collected from the Caoba site in Yuxi Prefecture in Yunnan (China) in July 2013. The sampled sites and their collection identifiers in Poland are presented in Fig. 1 and Table 1S (Supplementary material). Cantharellus cibarius mushrooms studied were exclusively from Poland. However, due to reports of elevated levels of REE in soils from Yunnan, a pool of C. minor from this region was also investigated. Although C. minor is a different species and does not occur in Europe, it is considered a unique material that could function as a positive control. Fresh fruiting bodies (from 4 to 309 per pool) were thoroughly cleaned of impurities, dehydrated at 65 °C to constant weight (dehydrator model MSG-01; MPM Product, Milanówek, Poland, and Ultra FD1000, Ezidri, Australia), hand-ground in a ceramic mortar and stored in clean, airtight polyethylene bags under dry conditions until chemical analysis.
Locations of C. cibarius sampling sites in Poland (see Table 2 for id and names of the sites)
Elemental analysis
Wet digestion was carried out on aliquots (0.5 ± 0.01 g) of dried and powdered mushroom samples with 8 mL solution of concentrated nitric acid (65%; Suprapur®) in a 1:1 ratio with deionized water and with the addition of 1 mL of hydrogen peroxide solution (30%, Suprapur®) in a high-pressure closed-vessel with the aid of Multiwave 3000 microwave-assisted mineralizer (Anton Paar). After digestion, the solution was quantitatively transferred to centrifuge tubes and made up to 25 mL with distilled water. The digests were stored at 4 °C until the analyses was performed. Analyses was performed by using inductively coupled plasma–quadrupole mass spectrometer (ICP-MS) model Elan DRCII model, PerkinElmer (Table 2S; Supplementary material). Before starting the analyses, the sensitivity of the spectrometer, the background value for mass 220, and the number of counts per second for two positive ions and oxides were checked in each measurement series, using the Elan DRC Setup/Stab/Masscal solution (PerkinElmer) containing 10 µg L−1 of Ba, and 1 µg L−1 each of Cd, Ce, Cu, In, Pb, Mg, Rh, and U in 0.5% solution of nitric acid (Dołęgowska and Migaszewski 2013; Migaszewski et al. 2016).
Working standard solutions as well as the Rh and Ir internal standards were prepared by the volumetric method on the day of analysis. Internal standards of 1 mg L−1 of Rh and 1 mg L−1 of Ir were added to each blank, standards, reference materials, and the samples. The volume of the added solutions of the internal standards was 100 µL of Rh and Ir for each 10 mL of medium analyzed. For the preparation of 1 mg L−1 internal standard solutions in 2% nitric acid, Rh and Ir solutions of 1000 mg L−1 (PerkinElmer) were used. In order to prepare the standard curve, six working solutions were prepared with the analyte concentrations in the range of 1–100 µg L−1. These solutions were prepared from the PerkinElmer standard containing 10 mg L−1 each of Ce, Dy, Er, Eu, Gd, Ho, La, Lu, Nd, Pr, Sm, Sc, Tb, Tm, Y, and Yb in 2% solution of nitric acid. The standards and samples were diluted with 2% solution of nitric acid and prepared on the day of the analysis by the gravimetric method using 65% solution of nitric acid and deionized water. The standard curves prepared for each analyte were characterized by high values of linear correlation coefficients in the range > 0.999–1 (Dołęgowska et al. 2013; Dołęgowska and Migaszewski 2013; Migaszewski et al. 2016).
Physical interferences were eliminated by using internal standards and diluting the samples. Spectral interferences were eliminated with the use of correction equations and, if possible, determination of different isotopes of the same element. The following reference materials were used to check the accuracy of the measurements: NIST-1573a Tomato leaves and IC-INCT-PVLT-6 Tobacco leaves. The recovery percentages for the elements whose concentrations were included in the certificate were as follows: La 98% and 107%; Ce 63%, Pr 74%, Nd 103%, Sm 104% and 78%, Eu 112%, Tb 76%, Gd 123%, Yb 55%.
Results and discussion
Scandium and yttrium concentrations
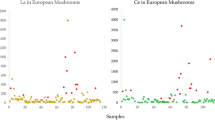
The content of Sc was < 1 µg kg−1 dw in C. cibarius from 20 sites and varied from 17 µg kg−1 dw for a composite from the Tatra Mountains (outskirts of the city Zakopane) to 27 µg kg−1 for the Tuchola Pinewoods in the Lubichowo forest district. C. minor from Yunnan showed a much higher Sc content of 66 µg kg−1 dw, compared to C. cibarius (Table 1).
In the earliest study of Sc in mushrooms (using instrumental neutron activation analysis, INAA), the element was detected in Lycoperdon pyriformis (Pear-shaped Puffball) at 480 µg kg−1 dw and in Scleroderma verucosa (Scaly Earthball) at 1300 µg kg−1 dw (Horowitz et al. 1974) — both results are substantially higher compared to the Cantharellus spp. investigated in this study (Table 1). INAA was used to determine Sc in a series of 115 individual mushrooms and the overall concentrations were in the range of 2 to 240 µg kg−1 dw (Řanda and Kučera 2004). For different species, this varied from 2.5 ± 0.3 µg kg−1 dw for Lycoperdon perlatum (also called Common Puffball, Gem-studded Puffball or Devil’s Snuffbox) to 76 ± 2 µg kg−1 dw for C. cibarius. In Cantharellus lutescens (Yellow Foot) and Cantharellus pallens (Pale Chanterelle) collected from the Bohemia (Czechia), the Sc contents were 44 ± 8 and 32 ± 1 µg kg−1 dw, respectively (Řanda and Kučera 2004). Saprotrophic Macrolepiota procera (field parasol or parasol mushroom) collected across Poland contained 28 ± 48 µg kg−1 dw (total < 1–160 µg kg−1 dw) Sc in the caps (determined using the Quadruple inductively coupled argon plasma–mass spectrometry analysis) and 28 ± 25 µg kg−1 dw (total 5.3–55 µg kg−1 dw) in the whole fruiting bodies (Falandysz et al. 2017).
In a more recent study, Sc was found at relatively higher concentrations in soil from the regions of Serbia (total range of 2000 to 13,000 µg kg−1 dw) but much lower concentrations were observed in M. procera from the same location, with mean values ranging from 14 ± 16 to 110 ± 70 to µg kg−1 dw (range < LOD to 240 µg kg−1 dw for caps, while in the stipes, concentrations ranged from 63 ± 43 to 80 ± 62 µg kg−1 dw) (Vukojević et al. 2019). Vukojević et al. demonstrated that Sc occurs minimally in M. procera compared to the soil substrate (where the mycelium grows). The BCF values varied from 0.013 to 0.017, showing bio-exclusion of the element in matured fruiting bodies of this species (Tyler 1982; Vukojević et al. 2019).
Yttrium was detected in all the composite samples of C. cibarius (median value of 22 µg kg−1 dw) and also in the C. minor collect from Yunnan (200 µg kg−1 dw) (Table 1). Similarly, as was observed for Sc, the C. cibarius originating from the outskirts of Zakopane and also from the Tuchola Pinewoods showed greater concentrations of Y (both values were 83 µg kg−1 dw). The Y concentrations for samples from sites at the Coastal Landscape Park and Augustowska Primeval Forest are 43 and 56 µg kg−1 dw, indicating more accumulation, far above the overall median value of 22 µg kg−1 dw (Table 1). Higher Sc and REY were observed in the C. minor compared to the C. cibarius (mostly by an order of magnitude), thereby confirming literature reports that these elements are higher in the bedrock of Yunnan and that the amounts translocated to the fruiting bodies also depends to a great extent on the amount in the topsoil substrate.
Compared to previous studies of Y in M. procera, a reported concentration of 74 ± 39 µg kg−1 dw in the caps and 110 ± 30 µg kg−1 dw in the whole mushrooms, indicated higher retention in the stipe and much slower translocation via the stipe to the cap (Falandysz et al. 2017). In a study of M. procera from Serbia, the element Y was found to be in the range of 9 ± 8 to 30 ± 27 µg kg−1 dw in the caps and, from 45 ± 39 to 74 ± 140 µg kg−1 dw in the stipes, with low BCFs of 0.009, indicating that this could have derived from the high levels of Y in the soil (3600 to 12,000 µg kg−1 dw; rounded) (Vukojević et al. 2019). The earliest study of Y in mushrooms (in Albatrellus pes-caprae; current name Scutiger pes-caprae, Goat’s Foot) in 2002 reported some results that were relatively elevated (range from < 50 to 2000 µg kg−1 dw; median of 270 µg kg−1 dw) (Stijve et al. 2002). It was later clarified that this was because of the difficulty in avoiding contamination with sand/soil particles during analysis (Stijve et al. 2004). A specimen of B. edulis from Germany was shown to contain about 18 µg kg−1 dw of Y, while a study of 10 composite samples (201 fruiting bodies) of the same species from Poland reported a concentration of 62 ± 76 µg kg−1 dw (Bau et al. 2018; Falandysz et al. 2022). Borovička et al. (2011) studied REE in 36 species of ectomycorrhizal (26 samples) and saprobic (25 samples) macro-fungi from unpolluted sites with differing bedrock geochemistry and reported concentrations that did not exceed 360 µg kg−1 dw. They also observed that their distribution more or less followed the trend observed in post-Archean shales and loess.
Concentration of 14 REE
The concentrations (µg kg−1 dw) of the 14 REE for the samples collected from locations in Poland varied widely ranging from < 1.0 to 250 (median of 35) for Ce; < 1.0 to 27 (2.3) for Pr; < 1.0 to 96 (9.2) for Nd; < 1.0 to 20 (3.1) for Sm; < 1.0 to 4.1 (0.5) for Eu; < 1.0 to 19 (2.2) for Gd; < 1.0 to 8.3 (0.5) for Tb; < 1.0 to 14 (2.0) for Dy; 1.0 to 1.4 (0.5) for Ho; 1.2 to 8.4 (2.5) for Er; < 1.0 to < 1.0 (0.5) for Tm; < 1.0 to 5.5 (0.5) for Yb; and < 1.0 to < 1.0 (0.5) for Lu. For the sample collected from Yunnan, the corresponding values were 480 for La, 35 for Ce, 2.3 for Pr, 9.2 for Nd, 3.1 for Sm, 0.5 for Eu, 56 for Gd, 2.5 for Tb, 34 for Dy, 5.7 Ho, 18 for Er, 1.6 for Tm, 11 for Yb, and < 1.0 for Lu. The sum of the 14 REE concentrations (ΣREE) which includes Sc and Y for the various locations investigated in Poland varied from 10.5 µg kg−1 dw for the Ciechocinek in Kujawy region to 592.8 µg kg−1 dw for Tatra Mountains, Zakopane, whereas that for the Yunnan sample was 2071.9 µg kg−1 dw (Table 1). Gałuszka et al. (2020) studied REE in plants collected in areas impacted by acid mine drainage in Poland and reported that sum of REE ranged from 0.069 to 28 mg kg−1 dw for one site and from 0.36 to 26.4 mg kg−1 dw for another. Studies have shown that moss accumulates more REE than plants. Gałuszka et al. (2020) observed REE for mosses that was 11 times higher than that for vascular plants. This may depend on unique features of mosses — they are non-vascular plants with simple tissues but rootless and featured by high surface-to-volume ratio, slow growth rate, aerial uptake of nutrients, and high ion-exchange capacity allowing them to bioconcentrate some atmospheric pollutants over long periods of time.
Concentrations of REE well above the median value were noted for locations such as Coastal Landscape Park (id 1), with REE of 204.9 µg kg−1 dw; Kaszubski Landscape Park (id 6) with 229.8 µg kg−1 dw, Tuchola Pinewoods in Lubichowo (id 9) with 504.8 µg kg−1 dw; Augustowska Primeval Forest (ID 15) with 260.8 µg kg−1 dw; Świetokrzyskie region in Włoszowa (id 21) with 206.2 µg kg−1 dw and Tatra Mountains, in Zakopane (id 22) with 592.8 µg kg−1 dw (Table 1).
The Baltic Sea marine sands at the southern coastal area are relatively rich in REE and have been reported as containing a “vast preponderance of light REE (LREE; La, Ce, Pr, Nd, Pm, Sm, Eu) among the rare earths” followed by Ce, La, and Nd and lastly by Y (Mikulski et al. 2016). On the other hand, the montane soils of the Świetokrzyskie and Tatra Mountains regions have a rocky background, while the Augustowska Primeval Forest region has deposits of sand and gravel, with some ore anomalies (Bońda et al. 2020). Forest areas of the Coastal Landscape Park, Kashubian Landscape Park and Bory Tucholskie (Lubichowo) have a sandy bedrock with a sandy topsoil, but the soil has not been investigated, and it has not been possible to confirm how the soils are responsible for the relatively higher REE observed in C. cibarius from these locations.
Distribution of REE concentrations in C. cibarius (median values) and in C. minor follows the Oddo-Harkins rule and shows characteristic “zigzag” pattern (Fig. 2). This concentration pattern of REE in C. cibarius fits with natural concentration pattern of REE in topsoil in Poland (Fig. 2).
Distribution pattern of REE in C. cibarius and topsoil in Poland (data on REE in topsoil adapted from Dołęgowska et al. 2013), and in C. minor
REE — Cantharellus mushrooms — human exposure
Balaram (2019) and Pagano et al. (2019) recently reviewed the available data on human risk from REE due to occupational and environmental exposures. Similarly, Doulgeridou et al. (2020) reviewed the risk from REE in plant-based foods. REE are not considered an essential compound in human nutrition. Lanthanum, which like other REE is known as a seeker for calcium (Ca), has been found to mimic (replace) Ca in some living function of bacteria Methylobacterium radiotolerans (Hibi et al. 2011). Because of the low or ultra-low occurrence of REE in foods including edible wild mushrooms, their dietary exposure is negligible. A few studies report data on REE which were considered substantially excessive — and the reasons for this have been discussed by Borovička et al. (2011), Zocher et al. (2018) and Falandysz (2023b).
The elements, Ce, La, and Nd were observed in higher concentrations in C. cibarius compared to other REE. Their contributions to the median value of REE concentrations was 42% for Ce, followed by La (35%) and then Nd (12%), and altogether, they accounted for 92% of the REE. The contribution of Ce, La, and Nd to REE is relatively high in specimens of C. cibarius from the Zakopane site from Poland (42, 24, and 16% respectively) and this is close to the proportions observed in C. minor which originated from a polymetallic soil background of Yunnan (45, 25, and 17%, respectively). China has rather high REE soil concentrations (Liang et al. 2005).
The mean concentration of summed REE plus Sc and Y determined in C. cibarius is 176 ± 167 µg kg−1 dw (rounded). This value is clearly low compared to values earlier reported for M. procera (mean concentration of 500 µg kg−1 dw in caps and 750 µg kg−1 dw in the whole fruiting bodies) or the whole B. edulis from Poland with 430 ± 430 µg kg−1 dw (median 310 µg kg−1 dw) (Falandysz et al. 2017, 2022). The amounts of the REE (plus Sc and Y) in C. cibarius mushrooms can be considered to be rather small (even negligible) from the point of view of food safety experts (food toxicologists and nutritionists). The reason can be that REE have some predilection to calcium (Ca) in organisms (Ascenzi et al. 2020), which is essential for fungi and undergoes homeostatic regulation (Lange and Peiter 2020). REE tend to accumulate in the bone structure (Chen and Zhu 2008). Vukojević et al. (2019) reported a positive correlation between lanthanides and Ca in mushrooms. Fruiting bodies of wild mushrooms are much richer in Ca (containing one to a few hundred mg kg−1 dw) than REE (Malinowski et al. 2021). It would be interesting to investigate further the possible relationship between REE and Ca in mushroom fruiting bodies, although at the present stage, the amount of reliable data on REE in mushrooms is too scarce. Calcium contents of C. cibarius collected in Poland and elsewhere (Table 2; including some batches of mushrooms in this study) were three orders of magnitude in excess of REE including Sc and Y (Table 1), i.e., in the range (median) from 100 to 270 mg kg−1 dw (60 pools of mushroom with 847 fruiting bodies) and from 200 to 520 mg kg−1 dw (60 pools of mushroom with 141 fruiting bodies) (Drewnowska and Falandysz 2015; Falandysz and Drewnowska 2015).
Shale normalized patterns of REE in Cantharellus mushrooms
Certain mushrooms were found to be more or less species-specific accumulators of some elements (e.g., Ag, As, Cd, Hg, Se, and V) and show good bioconcentration potential for these elements (Sácký et al. 2014; Komorowicz et al. 2019). No such phenomena could be identified thus far in the case of mushrooms and REE. As mentioned, the BCF values of REE determined for mushrooms were well below 1 and showing on bio-exclusion of REE by these organisms (Yoshida and Muramatsu 1997; Tyler 1982; Zocher et al. 2018; Mędyk and Falandysz 2022). It has been found that vegetation native to the sites impacted by acid mine drainage were good at bioconcentrating REE (Gałuszka and Migaszewski 2018; Gałuszka et al. 2020). No similar evidence could be found up to now in the case of edible wild mushrooms, which typically are collected from the forested areas and woodlands considered to be unpolluted, while mushrooms from anthropogenically impacted sites (metal smelters, metal refineries, legacy mine areas, cities etc.) can be contaminated with typical heavy metals (Zabowski et al. 1990; Árvay et al. 2014; Falandysz 2016, 2017).
As evidenced, ΣREE were found to be higher (i.e., above 200 µg kg−1 dw) in C. cibarius collected from six of the twenty-two studied locations (Table 1). Also, Y was elevated for these six locations whereas Sc was higher at two of the locations (for other location Sc was < 1 µg kg−1 dw) (Table 1). Considering that studies have shown that for mosses and vascular plants, a local abundance and bioavailability of REE in soil are important determinants for bioconcentration in above ground plant parts (Gałuszka and Migaszewski 2018; Gałuszka et al. 2020). It is also possible that the anomaly of REE in mushrooms could reflect both the REE natural occurrence in forest soil due to parent soil bedrock as well as potential releases from environmental pollution (especially if any anomalies in normalized pattern of the REE could be identified). This theoretically can be identified by the constructive analysis of the graphs of their normalized distribution in relation to their occurrence in shales with the aim of detecting and interpreting any hypothetical anomaly. Nevertheless, extensive research has been carried out in recent years and evidence has been found that distribution patterns of REE over Europe are entirely attributable to geology without any evidence of REE anthropogenic pollution (Fedele et al. 2008).
This study also examined the shale-normalized distribution of REY for a few collections of C. cibarius and C. minor (Fig. 3; REE and REY shale normalized pattern combinations in Figs. 1S-4S, Electronic Supplementary Material). The mean and median values of the REE concentrations in selected C. cibarius samples and in C. minor were normalized against the North American Shale Composite (NASC) and Post-Archean Australia Shales (PAAS) (Dołęgowska and Migaszewski 2013) as well as European Shale and World Shale (Bau et al. 2018; Migaszewski and Gałuszka 2019) (Fig. 3, Figs. 1S, 2S, 3S, and 4S, Tables 3S, 4S, and 5S, Electronic Supplementary Material; where the concentration was < 0.1 µg kg−1 dw a value of 0.5 µg kg−1 dw was used, this is roughly the limit of quantification).
NASC-, PAAS-, EUS- and WSH-shale normalized patterns of REY in C. cibarius from the Świetokrzyskie region in Włoszowa, Tatra Mountains in Zakopane, Coastal Landscape Park (Baltic Sea), Augustowska Primeval Forest and from all sites jointly in Poland (based on median concentration values), and in C. minor from Yunnan in China
The REE are often grouped into light REE (La, Ce, Pr, and Nd; LREE), middle REE (Sm, Eu, Gd, Tb, and Dy; MREE), and the heavy REE (Ho, Er, Tm, Yb, and Lu; HREE). Anomalies were observed in the REY plots among the MREE and more predominantly in HREE (Figs. 1S, 2S, and 3S) for all the sites considered except for the C minor from Yunnan China (cf. Figure 4S; the normal and log-normalized plots). For the C minor from Yunnan China, anomalies were observed more for Tb and possibly also for Lu. Tb negative anomaly was also observed for the Tuchola Pinewood. Considering normalization of the median values for all sites (Fig. 4Sg’), the highest anomaly (negative) was observed for Yb, similar to the pattern observed for the Świetokrzyskie region in Włoszowa (Fig. 2Sa´), the Baltic Sea Coastal Landscape Park (Fig. 2Sc´) and Kaszubski Landscape Park. Kaszubski Landscape Park (Fig. 2Sd´).
Conclusion
Median REE concentrations determined in 22 collective samples of C. cibarius collected in Poland indicate their negligible content, and thus no risk to consumers of this popular wild mushroom. The pooled sample of C. minor from Yunnan was richer in REE than C. cibarius from Poland, and this may be a good reason for more extensive research on REE in Yunnan mushrooms.
Data availability
Not applicable.
Change history
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11356-023-25961-2
References
Andersson M, Reimanna C, Flem B, Englmaier P, Fabian K (2018) Element distribution in Lactarius rufus in comparison to the underlying substrate along a transect in southern Norway. Appl Geochem 97:61–70
Aruguete DM, Altstad JH, Mueller GM (1998) Accumulation of several heavy metals and lanthanides in mushrooms (Agaricales) from the Chicago region. Sci Total Environ 224:43–56
Árvay J, Tomáš J, Hauptvogl M, Kopernická M, Kováčik A, Bajčan D, Massányi P (2014) Contamination of wild-grown edible mushrooms by heavy metals in a former mercury-mining area. J Environ Sci Health Part B 49:815–827
Ascenzi P, Bettinelli M, Boffi A, Botta M, De Simone G, Luchinat C, Marengo E, Mei H (2020) Aime S (2020) Rare earth elements (REE) in biology and medicine. Rendiconti Lincei Scienze Fisiche e Naturali 31:821–833. https://doi.org/10.1007/s12210-020-00930-w
Bakaytis VI, Golub OV, Miller YY (2021) Fresh and processed wild Cantharellus cibarius L. growing in West Siberia: food value. Foods Raw Mat 9:234–243
Balaram V (2019) Rare earth elements: a review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci Front 10:1285–1303
Bau M, Schmidta K, Pack A, Bendel V, Kraemera D (2018) The European Shale: an improved data set for normalisation of rare earth element and yttrium concentrations in environmental and biological samples from Europe. Appl Geochem 90:142–149
Bengtsson G (2021) Hypothetical soil thresholds for biological effects of rare earth elements. Jagric Sci 13:1–40. https://doi.org/10.5539/jas.v13n5p1
Bielawski L, Kannan K, Gucia M, Lipka K, Brzostowski A (2002) Mercury in wild mushrooms and underlying soil substrate from the great lakes land in Poland. J Environ Monit 4:473–476
Bońda R et al (2020) Bilans zasobów złóż kopalin w Polsce. In: Szuflicki M, Malon A, Tymiński A (eds) Państwowy Instytut Geologiczny. Państwowy Instytut Badawczy, Warszawa
Borovička J, Kubrova J, Rohovec J, Řanda Z, Dunn CE (2011) Uranium, thorium and rare earth elements in macrofungi: what are the genuine concentrations? Biometals 24:837–845
Chen Z, Zhu X (2008) Accumulation of rare earth elements in bone and its toxicity and potential hazard to health. J Ecol Rural Environ 24:88–91
Dołęgowska S, Migaszewski ZM (2013) Anomalous concentrations of rare earth elements in the moss-soil system from south - central Poland. Environ Pollut 178:33–40
Dołęgowska S, Migaszewski ZM, Michalik A (2013) Hylocomium splendens (Hedw.) B.S.G. and Pleurozium schreberi (Brid.) Mitt. as trace element bioindicators: statistical comparison of bioaccumulative properties. J Environ Sci 25:340–347
Doulgeridou A, Amlund H, Sloth JJ, Hansen M (2020) review of potentially toxic rare earth elements, thallium and tellurium in plant-based foods. EFSA J 18(1):e181101. https://doi.org/10.2903/j.efsa.2020.e181101
Drewnowska M, Falandysz J (2015) Investigation on minerals composition and accumulation by popular edible mushroom Common Chanterelle (Cantharellus cibarius). Ecotoxicol Environ Saf 113:9–17
Drewnowska M, Hanć A, Barałkiewicz D, Falandysz J (2017) Pickling of chanterelle Cantharellus cibarius mushrooms highly reduce cadmium contamination. Environ Sci Pollut Res 24:21733–21738
Falandysz J (2016) Mercury bio-extraction by fungus Coprinus comatus: a possible bioindicator and mycoremediator of polluted soils. Environmental Science and Pollution Research, 23, 7444-7451. doi.10.1007/s11356-015-5971-8.
Falandysz J (2017) Mercury accumulation of three Lactarius mushroom species. Food Chem 214:96–101. https://doi.org/10.1016/j.foodchem.2016.07.062
Falandysz J (2023a) Comment on “worldwide basket survey of multielemental composition of white button mushroom Agaricus bisporus”: the credibility of the concentration data reported for REE are questioned – are they reliable enough to be included in the database on nutrients in mushrooms? Chemosphere 310:136857
Falandysz J (2023b) Letter to the editor: Comment on “multiannual monitoring (1974–2019) of rare earth elements in wild growing edible mushroom species in Polish forests” by Siwulski et al A recurring question - what are the real concentrations and patterns of REE in mushrooms? Chemosphere 312:137219. https://doi.org/10.1016/j.chemosphere.2020.127173
Falandysz J (2023c) Comment on “Screening the multi-element content of Pleurotus mushroom species using inductively coupled plasma optical emission spectrometer (ICP-OES)”. Food Anal Methods. https://doi.org/10.1007/s12161-022-02440-x
Falandysz J, Drewnowska M (2015) Macro and trace elements in common Chanterelle (Cantharellus cibarius) mushroom from the European background areas in Poland: composition, accumulation, dietary exposure and data review for species. J Environ Sci Health Part B 50:374–387
Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki S (2001) ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit Contam 18:503–513
Falandysz J, Zalewska T, Apanel A, Drewnowska M, Kluza K (2016) Evaluation of the activity concentrations of 137Cs and 40K in some Chanterelle mushrooms from Poland and China. Environ Sci Pollut Res 23:20039–20048
Falandysz J, Sapkota A, Mędyk M, Feng X (2017) Rare earth elements in parasol mushroom Macrolepiota procera. Food Chem 221:24–28
Falandysz J, Nnorom IC, Mędyk M (2022) Rare earth elements in Boletus edulis (King Bolete) mushrooms from lowland and montane areas in Poland. Int J Environ Res Public Health 19:8948
Fedele L, Plant JA, De Vivo B, Lima A (2008) The rare earth element distribution over Europe: geogenic and anthropogenic sources. Geochemistry: Exploration. Environ Anal 8:3–18
Franus W, Wiatros-Motyka MM, Wdowin M (2015) Coal fly ash as a resource for rare earth elements. Environ Sci Pollut Res 22:9464–9474
Gałuszka A, Migaszewski ZM (2018) Extremely high levels of trace elements in aerial parts of plants naturally growing in the Wiśniówka acid mine drainage area (south-central Poland). In: Wolkersdorfer C, Sartz L, Weber A, Burgess J, Tremblay G (eds) Risk to Opportunity, 2nd edn. ICARD/IMWA 2018, Pretoria, pp 598–603
Gałuszka A, Migaszewski ZM, Pelc A, Trembaczowski A, Dołęgowska S, Michalik A (2020) Trace elements and stable sulfur isotopes in plants of acid mine drainage area: implications for revegetation of degraded land. J Environ Sci (china) 94:128–136
Grawunder A, Gube M (2018) Element distribution in fruiting bodies of Lactarius pubescens with focus on rare earth elements. Chemosphere 208:614–625
Hibi Y, Asai K, Arafuka H, Hamajima M, Iwama T, Kawai K (2011) Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J Biosci Bioeng 111:547–549
Horowitz CT, Schock HH, Horovltz-Kisimova LA (1974) The content of scandium, thorium, silver, and other trace elements in different plant species. Plant Soil 40:397–403
Ichihashi H, Morita H, Tatsukawa R (1992) Rare earth elements (REEs) in naturally grown plants in relation to their variation in soils. Environ Pollut 76:157–162
Kaegi R, Gogos A, Voegelin A, Hug SJ, Winkel LHE, Buserd AM, Berg M (2021) Quantification of individual rare earth elements from industrial sources in sewage sludge. Water Res X 11:100092
Komorowicz I, Hanć A, Lorenc W, Barałkiewicz D, Falandysz J, Wang Y (2019) Arsenic speciation in mushrooms using dimensional chromatography coupled to ICP-MS detector. Chemosphere 233:223–233
Kuo M (2006) Cantharellus minor. Mushroom Expert Com. https://www.mushroomexpert.com/cantharellus_minor.html. Accessed 30 June 2022
Lange M, Peiter E (2020) Calcium transport proteins in fungi: the phylogenetic diversity of their relevance for growth, virulence, and stress resistance. Front Microbiol 10:3100. https://doi.org/10.3389/fmicb.2019.03100
Liang T, Zhang S, Wang L, Kung H, Wang Y, Hu A, Ding S (2005) Environmental biogeochemical behaviors of rare earth elements in soil–plant systems. Environ Geochem Health 27:301–311
Malinowski R, Sotek Z, Stasińska M, Malinowska K, Radke P, Malinowska A (2021) Bioaccumulation of macronutrients in edible mushrooms in various habitat conditions of NW Poland—role in the human diet. Int J Environ Res Public Health 18:8881
Markert B, Li ZD (1991) Natural background concentrations of rare earth elements in a forest ecosystem. Sci Total Environ 103:27–35
Mędyk M, Falandysz J (2022) Occurrence, bio-concentration and distribution of rare earth elements in wild mushrooms. Sci Total Environ 851(Part1):158159
Mędyk M, Grembecka M, Brzezicha-Cirocka J, Falandysz J (2017) Bio- and toxic elements in mushrooms from the city of Umeå and outskirts, Sweden. J Environ Sci Health Part B 52:577–583
Mędyk M, Treu R, Falandysz J (2020) Accumulation of minerals by Leccinum scabrum from two large forested areas in Central Europe: Notecka Wilderness and Tuchola Forest (Pinewoods). Chem Biodiversity 17:e2000264
Migaszewski ZM, Gałuszka A (2015) The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: a review. Crit Rev Environ Sci Technol 45:429–471
Migaszewski ZM, Gałuszka A (2019) Pierwiastki ziem rzadkich w kwaśnych wodach kopalnianych – zarys problematyki. Przegląd Geologiczny 67:105–114. https://doi.org/10.7306/2019.2
Migaszewski ZM, Gałuszka A, Dołęgowska S (2016) Rare earth and trace element signatures for assessing an impact of rock mining and processing on the environment: Wiśniówka case study, south-central Poland. Environ Sci Poll Res 23:1–17
Mikulski SZ, Kramarska R, Zieliński G (2016) Rare earth elements pilot studies of the Baltic marine sands enriched in heavy minerals. Gosp Sur Miner-Min Res Manage 32:5–28
Mirończuk-Chodakowska I, Socha K, Zujko M, Terlikowska KM, Borawska MH, Witkowska AM (2019) Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of Green Lungs of Poland: nutritional and toxicological implications. Int J Environ Res Public Health 16:3614
Mleczek M, Siwulski M, Stuper-Szablewska K, Sobieralski K, Magdziak Z, Goliński P (2013) Accumulation of elements by edible mushroom species II. A comparison of aluminium, barium and nutritional element contents. J Environ Sci Health Part B 48:308–317
Pagano G, Thomas PhJ, Nunzio AD, Trifuoggi M (2019) Human exposures to rare earth elements: Present knowledge and research prospects. Environ Res 171:493–500
Pelkonen R, Alfthan G, Järvinen O (2008) Element concentrations in wild edible mushrooms in Finland. The Finnish environment 25|2008. Finnish Environment Institute, Helsinki
Prohaska T, Hann S, Latkoczy C, Stingeder G (1999) Determination of rare earth elements U and Th in environmental samples by inductively coupled plasma double focusing sector field mass spectrometry (ICP-SMS). J Anal at Spectrom 14:1–8
Řanda Z, Kučera J (2004) Trace elements in higher fungi (mushrooms) determined by activation analysis. J Radioanal Nucl Chem 259:99–107
Řanda Z, Kučera J, Mizera J (2005) Possibilities of the short-term thermal and epithermal neutron activation for analysis of macromycetes (mushrooms). J Radioanal Nucl Chem 264:67–76
Saba M, Falandysz J, Loganathan B (2020) Accumulation pattern of inorganic elements in scaly tooth mushroom (Sarcodon imbricatus) from Northern Poland. Chem Biodivers 17:e2000167
Sácký J, Leonhardt T, Borovička J, Gryndler M, Briksí A, Kotrba P (2014) Intracellular sequestration of zinc, cadmium and silver in Hebeloma mesophaeum and characterization of its metallothionein genes. Fungal Gen Biol 64:3–14
Stijve T, Goessler Dupuy WG (2004) lnfluence of soil particles on concentrations of aluminium, iron, calcium and other metals in mushrooms. Deuts Lebensm Runds 100:10–13
Stijve T, Andrey D, Lucchini GF, Goessler W (2002) Lanthadines and other less common metals in mushrooms. Deutsch Lebensm Runds 98:82–87
Tyler G (1982) Accumulation and exclusion of metals in Collybia peronata and Amanita rubescens. Trans Br Mycol Soc 79:239–245
Venturella G, Gargano ML, Compagno R, Saitta A, Alaimo MG (2014) The mineral contents of some Boletaceae species from Sicily (Southern Italy). J AOAC Int 97:612–623
Volk AA, Gorbunov AV, Gundrina SF, Revich B, Frontasyeva MV, Pal CS (1990) Phosphorus fertilizer production as a source of rare-earth elements pollution of the environment. Sci Total Environ 95:141–148
Varo P, Lähelmä O, Nuurtamo M, Saari E, Koivistoinen P (1980) Mineral element composition of Finnish foods. VII. Potato, vegetables, fruits, berries, nuts and mushrooms. Acta Agric Scand Supplement 22:89–113
Vukojević V, Durdić S, Stefanović V, Trifković J, Čakmak D, Perović V, Mutić J (2019) Scandium, yttrium, and lanthanide contents in soil from Serbia and their accumulation in the mushroom Macrolepiota procera (Scop.). Environ Sci Pollut Res 26:5422–5434
Yoshida S, Muramatsu Y (1997) Determination of major and trace elements in mushroom, plant and soil samples collected from Japanese forests. Int J Environ Anal Chem 67:49–58
Zabowski D, Zasoski RJ, Littke W, Ammirati J (1990) Metal content of fungal sporocarps from urban, rural, and sludge-treated sites. J Environ Qual 19:372–377
Zawisza B, Pytlakowska K, Feist B, Polowniak M, Kita A, Sitko R (2011) Determination of rare earth elements by spectroscopic techniques: a review. J Anal at Spectrom 26:2373–2390
Zepf V (2013) Rare earth elements. A new approach to the Nexus of supply, demand and use: exemplified along the use of neodymium in permanent magnets. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35458-8
Zhang J, Falandysz J, Hanć A, Lorenc W, Wang Y, Barałkiewicz D (2022) Occurrence, distribution, and associations of essential and non-essential elements in the medicinal and edible fungus “Fuling” from southern China. Sci Total Environ 831:155011
Zocher A-L, Kraemer D, Merschel G, Bau M (2018) Distribution of major and trace elements in the bolete mushroom Suillus luteus and the bioavailability of rare earth elements. Chem Geol 483:491–500
Acknowledgements
The support kindly provided by Professor Zdzisław Maksymilian Migaszewski from the Jan Kochanowski University in Kielce (Poland) is acknowledged.
Author information
Authors and Affiliations
Contributions
MM: resources, investigation, formal analysis, data curation, visualization, help with Quaerenda, draft and review. JF: conceptualization, resources, investigation, funding acquisition, formal analysis, data curation, writing — original draft, and review and editing. ICN: data curation, visualization, help with Quaerenda, review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable. This manuscript does not contain any individual person’s data in any form.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The correct family name of the last Author is Nnorom.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mędyk, M., Falandysz, J. & Nnorom, I.C. Scandium, yttrium, and lanthanide occurrence in Cantharellus cibarius and C. minor mushrooms. Environ Sci Pollut Res 30, 41473–41484 (2023). https://doi.org/10.1007/s11356-023-25210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25210-6