Abstract
Ivermectin is the medication of choice for treating human onchocerciasis and is used in veterinary medicine to treat a variety of ectoparasites and endoparasites. This study was designed to investigate the effects of zinc nanoparticles (ZnNPs) on the fertility of male rabbits exposed to experimental ivermectin (IVM) intoxication. A total of 72 mature male rabbits were equally divided into 4 groups (n = 18). The first group (CTR) served as control; the second group (IVM) received subcutaneous injection of IVM (0.2 mg/kg body weight); the third group (ZnNPs) fed on zinc nanoparticles (60 mg/kg diet); and the fourth group (ZnNPs + IVM) were administered IVM and zinc nanoparticles at the same doses. The experiment lasted for 9 weeks. Results revealed that IVM-intoxicated rabbits showed impaired growth performance parameters, including body weight, total body weight gain (TBWG), total feed intake (TFI), and feed conversion ratio (FCR). Moreover, carcass characteristic and fertility parameters (including semen quality parameters and testosterone levels) were also impaired after IVM administration. Additionally, testicular malondialdehyde (MDA) and antioxidant (reduced glutathione, superoxide dismutase, catalase) levels as well as the histopathology and immunohistochemical expression of caspase 3 and PCNA in the testes and epididymis were detrimentally affected. On the contrary, ZnNP administration efficiently improved most of these parameters in IVM-intoxicated rabbits. In conclusion, ZnNPs exhibited promising ability for improving the growth and fertility status of rabbits and reducing the deleterious effects of IVM possibly through the suppression of apoptotic and oxidative pathways.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Commercial rabbits have recently gained considerable attention due to their high prolificacy and rapid growth rate compared to broiler chicken (Azazi et al. 2018). Rabbits are an important source of protein for humans because of its high quality and low fat and cholesterol content (PARA et al., 2015). With the increase in rabbit production, the need for animal supplements has become a necessary part of their daily diet. As such, studies into many areas of rabbit production are urgently needed (Abdel-Wareth et al. 2015; Al-Sagheer et al. 2017).
In commercial rabbit production, parasitic infestation, particularly by Sarcoptes scabiei, which causes mange, has been a major concern (El-Ashram et al. 2020). Ivermectin (IVM) is a drug extensively used to treat and prevent sarcoptic mange, a dangerous condition with serious health implications, including rabbit death (Joshi et al. 2021). This particular drug is an acaricide and anthelmintic developed from avermectin B1, which originates from Streptomyces avermitilis. The subcutaneous route has been identified as the most efficient and recommended route of administration, promoting better absorption than the oral and topical routes (Khan Sharun et al. 2019).
IVM is generally well tolerated in mammals (Johnson-Arbor 2022); however, in female rabbits, repeated IVM doses caused pathological alterations in hepatic tissue, including vacuolation of hepatocytes and fibrosis (Al-Jassim et al. 2015). In male rats, therapeutic and double therapeutic doses reduced the total sperm count and induced sperm mortality, as well as pathological abnormalities in the liver, kidneys, and testis. Congestion of blood vessels, degenerative changes (e.g., vacuolar, hydropic, and even necrotic changes), and functional problems of the liver and kidneys are some of the pathogenic changes (Elzoghby et al. 2015). Injecting IVM every 2 weeks has been the standard prophylactic regimen used by rabbit breeders. In addition, IVM is reported to cause excessive generation of reactive oxygen species from the mitochondria, which could potentially interfere with the proper release of cytochrome c, which subsequent activation of caspase-3 and induction of cell apoptosis (Ali et al., 2017). Moreover, it can reduce the expression of proliferating cell nuclear antigen (PCNA), which plays a pivotal role in the S-phase of the cell cycle via DNA synthesis and replication (Strzalka and Ziemienowicz 2011). Therefore, the reduction in PCNA expression may promote suppression of DNA synthesis with subsequent DNA damage (Moshari et al. 2017).
Several metabolic processes and physiological functions of animals rely heavily on trace minerals (Underwood 2012). Zinc (Zn) is the mammalian body’s second most abundant trace element (Dosoky et al. 2022). It cannot be stored in the body and must be consumed on a regular basis to meet physiological requirements (Fairweather-Tait and de Sesmaisons 2019). Zn is an essential component of around 300 enzymes involved in the production and degradation of proteins, lipids, carbohydrates, and nucleic acids, as well as in the metabolism of other micronutrients (Abd El-Hack et al. 2017). It is also a required component of the superoxide dismutase (SOD) enzyme, which plays an important role in the antioxidant defense system (Azad et al. 2017). Additionally, Zn is essential for polynucleotide transcription, which leads to genetic expression, and plays an important role in immune system function, affecting humoral and cellular immunity (Chasapis et al. 2020). Zn boosts T cell production by increasing thymus gland secretion of thymulin. Hence, Zn deficiency results in thymus malfunction, which has a major impact on proper immune function (Mocchegiani et al. 2013). Zn is also required for optimal human physiological activities, such as regular growth and reproduction (Swain et al. 2016).
Recently, several studies have concluded that animals fed zinc nanoparticles (ZnNPs) had better growth, reproduction, and immunity compared to those who were not. To date, however, no available studies have investigated the impact of ZnNPs on the side effects of IVM. Therefore, the present study aimed to determine the possible impact of ZnNPs on the growth and fertility of male rabbits receiving IVM treatment, focusing specifically on the possible mechanisms of action.
Materials and methods
Ethical statement
The Institutional Animal Care and Use Committee of University of Alexandria approved the experimental protocol used in this study (Permit #2021/013/97).
Chemicals
Zn oxide 99.99% (ZnO powder; containing 80.32% Zn, as an inorganic form of Zn) was purchased as a commercial product from Sigma Company, Egypt. Nano-Zn oxide 97% (nZnO powder; containing 77.92% Zn, as a nano-form of Zn) was purchased as a commercial product from Sigma Company, Egypt. The size of Zn oxide nanoparticles was ˂ 50 nm according to the manufacturer company. IVM was purchased from Alfasan International BV Company, the Netherlands.
Experimental design
A total number of 72 V-line male rabbits (3 months old) were used in this experiment. Rabbits were individually reared in batteries (width × length × height; 44 cm × 50 cm × 35 cm, respectively) of galvanized wire net, equipped with an automatic drinker and a manual feeder. Rabbits were reared in an open house system (naturally ventilated room by windows and ceiling fans). The temperature was adjusted to 19–23 °C. Relative humidity was nearly 60% with a 16-h light and 8-h dark cycle. Fresh tap water was continuously available for consumption via stainless steel nipples located inside each cage. Rabbits were acclimated for 2 weeks before the beginning of experimental procedures.
After acclimatation, animals were divided into four groups (18 bucks/group), and each group was subdivided into three replicates (6 bucks/replicate). Bucks were fed a balanced basal diet containing all the required nutrients according to NRC (1977) (Table 1). The treatment groups were as follows: (1) the first group served as control (CTR) and received a basal diet without ivermectin (IVM) injection; (2) the second group (IVM) received a basal diet with IVM injection; (3) the third group (ZnNPs) received a basal diet with inorganic Zn replacement through ZnNPs (60 mg/kg diet) without IVM injection; and (4) the fourth group (ZnNPs + IVM) received a basal diet with inorganic Zn replacement through ZnNPs (60 mg/kg diet) with IVM injection. IVM was administrated via subcutaneous injection at dose of 0.2 mg/kg body weight (BW). IVM injection was initiated when bucks were 14 weeks old and repeated weekly for five consecutive weeks until 19 weeks old. A pelleted diet was provided ad libitum for animals during the whole experiment.
Growth performance parameters
Individual live BWs were recorded as the initial BW at 12 weeks old and every 2 weeks until the end of the experiment (the final BW was obtained at 21 weeks old). Total weight gain and total feed intake were recorded, after which the average feed conversion ratio, that is, the amount of total feed intake/total body gain, was calculated.
Assessments of carcass characteristics
For the assessment of carcass characteristics, nine rabbits from each treatment were randomly selected. Rabbits were weighed in a fasted state before slaughtering to determine the live body weight. After the slaughtered rabbits were bled, the skin, genitals, urinary bladder, gastrointestinal tract, and the distal part of the legs were removed. Hot carcasses (with the head, thoracic cage organs, liver, kidneys, and perirenal and scapular fat) were weighed. The dressing percentage and the ratio of thigh, skin (with head skin), skin (without head skin), head with skin, head without skin, heart, lung, liver, spleen, abdominal fat, and kidney relative to the live BW were calculated.
Dimensions and relative weight of the reproductive organs
Organ dimensions (scrotum, penile length, and testicular circumference, length, and width) were measured after slaughter. Circumference was measured using a measuring tape, whereas testicular length was measured using an obstetrical pelvimeter.
Epididymal sperm preparation
Immediately after slaughtering, the animals were dissected and the epididymis was collected as quickly as possible and placed in a clean Petri plate. Thereafter, the cauda epididymis was separated from the whole epididymis, cut into several pieces, immersed in 3 mL pre-warmed phosphate buffer saline (PBS) solution, and incubated for 10 min at 37 °C to allow for sperm release from the epididymal lumen (Mangoli et al. 2013). The sperm count was evaluated using a hemocytometer chamber (count × 106) at 40 × magnification using light microscopy (Olympus Co., Tokyo, Japan). The percentages of progressive motility and viability were evaluated for at least 200 spermatozoa from each buck. Assessment of motile sperm at the warm stage showed progressive forward movement under 100 × magnification using a light microscope. Assessment of live and dead sperm was performed by counting 200 sperm cells using an eosin-nigrosin staining mixture. Complete or partial purple-stained sperm cells were considered non-viable, whereas non-stained sperm cells were considered viable.
Plasma testosterone measurement
Using an indirect enzyme immunoassay assay kit (Monobind, 100 North point Drive, Lake Forest, CA), plasma testosterone levels were estimated following the methods described by Tietz (1995).
Antioxidant indicators
At the end of the experimental period, rabbits were slaughtered and dissected. The testes were carefully removed, cut into small pieces, and preserved at − 20 °C for further analysis of Zn, reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA). Testicular Zn (CAT. Zn 21 20, Biodiagnostic), SOD (CAT. SD 25 21, Biodiagnostic), and CAT (Cat. CA 2517, Biodiagnostic) were assessed using spectrophotometric procedures (Hitachi spectrophotometer, Tokyo, Japan) with commercially available kits (Biodiagnostic Co., Dokki, Giza, Egypt) according to the manufacturer’s instructions. GSH (CAT. No. GR 25 11, Biodiagnostic) and MDA (Cat. MD 2529, Biodiagnostic) testicular contents were examined using the colorimetric method according to Beutler et al. (1963) and Okhawa et al. (1979), respectively.
Histopathological study
Immediately after slaughtering, specimens were collected from testes of control and treated bucks. The collected samples were washed and immersed for 48 h in a 10% neutral-buffered formalin solution for fixation. Fixed samples were prepared using the routine paraffin-embedding technique (Bancroft 2013). Briefly, fixed samples were dehydrated in ascending grades of ethanol, cleared using several changes of xylene, prepared in paraffin blocks, and microtomed into 3–5-µm-thick sections. Prepared sections were routinely stained with hematoxylin and eosin (H & E staining). Thereafter, blinded examination and image capture were performed by an experienced pathologist. Representative photomicrographs were obtained using a digital camera (Leica EC3; Leica, Germany) connected to a microscope (Leica DM500). Scoring for spermatogenesis was performed via Johnsen’s scoring system. The presence or absence of the primary cell types and/or lesions was scored from 1 to 10 according to Johnsen (1970) and Hassan et al. (2019). Twenty seminiferous tubules were randomly selected in each cross-section and scored under a light microscope (× 400); the mean score was determined for each group.
Immunohistochemical study
For immunohistochemical evaluation, each paraffin block was cut into several 4-µm-thick sections and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was done in citrate-buffered saline (0.01 mol/L, pH 6.0), and endogenous peroxidase activity was quenched in phosphate-buffered saline with H2O2 0.3% (v/v) (PBS). Thereafter, sections were incubated for 1 h with 10% (v/v) normal goat serum to block non-specific immunologic reagent binding, and tissue sections were incubated overnight at 4 °C with anti-Caspase-3 antibody rabbit monoclonal [EPR18297] (Cat, ab184787, Abcam, Cambridge, UK) and Anti-PCNA Mouse monoclonal antibody [24/PCNA] (Cat, ab280088 Abcam, Cambridge, UK). Afterwards, sections were washed in PBS and treated for 60 min with biotin-conjugated goat anti-rabbit IgG antiserum (Histofine kit, Nichirei Corporation, Japan). The sections were then rewashed in PBS and treated for 30 min with streptavidin-peroxidase conjugate (Histofine kit, Nichirei Corporation, Japan). The streptavidin–biotin complex was observed for 3 min with a 3,3′-diaminobenzidine tetrahydrochloride (DAB)-H2O2 solution. Finally, Mayer’s hematoxylin solution was used to counterstain the sections. Several original micrographs were obtained from five high-power fields/sections/organs at random and used for quantitative histomorphometric examination of immunostaining. Caspase-3 and PCNA positive brown color cells were counted in each micrograph (HPF, × 40) using manual computer-assisted cell counting (ImageJ plug-in-cell counter.jar) with ImageJ (v1.46 r, NIH, Bethesda, MD, USA) (Schneider et al. 2012) as reported by Powell et al. (2014). For each group, the mean count of immunological positive cells was computed and analyzed using non-parametric statistics (Saleh et al. 2020).
Statistical analyses
Statistical analyses of the data were performed using SAS software (SAS 2014). One-way analysis of variance (ANOVA) was used for data analysis. Duncan’s test was used when treatment effects were significant. The overall significance level was set at P < 0.05. All values were expressed as mean ± standard error. Histomorphometric analysis of caspase-3 and PCNA immune expressions were analyzed using the non-parametric analysis using Kruskal–Wallis test to assess the significance between mean scores obtained from Wilcoxon rank-sum test.
Results
Growth performance
The effects of dietary inorganic Zn replacement through ZnNPs with or without IVM injection on the growth performance of buck rabbits are presented in Table 2. The statistical analysis of our data revealed no significant differences between the live BW of different experimental groups at baseline and 2 weeks later (12 and 14 weeks old). However, rabbits in the IVM and IVM + ZnNPs groups showed a significant (P < 0.0001) reduction in live BW at 18, 20, and 21 weeks old compared to the control group. In addition, IVM-treated rabbits had significantly lower total BW gain (TBWG) and total feed intake (TFI) (P < 0.0001) compared to control group, which caused worst FCR. Conversely, rabbits in the ZnNPs group had significantly higher TFI (P < 0.0001) compared to control rabbits. Interestingly, rabbits in the ZnNPs + IVM group showed significant greater improvement in live BW at 16, 18, and 20 weeks old and TFI, with a subsequent improvement in FCR, compared to the IVM group.
Carcass quality
The effects of dietary ZnNP supplementation with or without IVM injection on dressing percentage and carcass quality of buck rabbits are shown in Table 3. Tabulated results revealed that the dressing percentage was significantly increased (P < 0.0001) in the ZnNPs group and significantly lower (P < 0.0001) in the IVM group compared to the control group. However, the relative weight of the thigh was significantly improved (P < 0.0001) in the ZnNPs group compared to the control group. Conversely, skin (with or without head skin) and abdominal fat showed no significant change (P < 0.0001) compared to the control group. In addition, the relative weights of the head with skin, heart, and lungs were significantly lower (P < 0.0001) compared to the control group. The relative weights of the head without skin and spleen were significantly decreased in IVM group (P < 0.0001) compared to the control group. Additionally, the relative weights of the liver were significantly greater (P < 0.0001) in the IVM group and significantly lower (P < 0.0001) in ZnNPs and ZnNPs + IVM groups compared to control group. The relative weight of the kidney was significantly lower (P < 0.0001) in the IVM and ZnNPs + IVM groups compared to the control group.
Dimensions and relative weight of reproductive organs
Data in Table 4 shows that the diameter of the scrotum was significantly lower in the IVM group and significantly greater (P < 0.0001) in ZnNPs group compared to the control group. However, the penile length of the different groups differed significantly (P < 0.0001) compared to that of the control group, with ZnNPs group and CTR group having the greatest and least values (1.95 and 1.50 cm, respectively). In addition, testicular circumference and length were significantly lower (P < 0.0001) in all treatment groups compared to the control group. However, testicular width was significantly higher (P < 0.0001) in ZnNPs + IVM compared to control group.
Conversely, the weight of the testis, epididymis, and pituitary gland did not differ significantly among groups. However, the weight of the accessory glands was significantly increased (P < 0.0001) in ZnNPs and ZnNPs + IVM group compared to the control group (Table 4).
Semen quality parameters
As shown in Table 4, sperm motility and livability were significantly higher in ZnNPs group compared to the control group. However, sperm concentrations were significantly lower in the IVM and ZnNPs compared to the control group.
Testosterone concentration and age at puberty
In Table 5, serum testosterone concentrations at 16 weeks old were significantly (P < 0.0026) higher in ZnNPs group compared to the control group. Meanwhile, serum testosterone concentration at 18 weeks old was significantly higher (P < 0.0001) in ZnNPs and ZnNPs + IVM groups than in the control group. At 20 weeks old, serum testosterone concentrations did not significantly differ (P < 0.0001) among the groups.
Additionally, the age at puberty was recorded for all groups; ZnNPs and Zn-NPs + IVM groups reached puberty (around 18 weeks of age) significantly earlier (P < 0.0066) than the other groups. Meanwhile, the CTR and IVM groups reached puberty at 19 and 20 weeks old, respectively (Table 5).
Antioxidant indicators
As shown in Table 6, dietary replacement of inorganic Zn with ZnNPs significantly enhanced testicular Zn concentrations as compared to control. The CAT enzyme was significantly lower in the IVM group compared to the control group. In contrast, GSH concentrations were significantly greater in the ZnNPs group compared to the control group. Interestingly, SOD concentrations were significantly higher in the ZnNPs and ZnNPs + IVM groups compared to the control group. Meanwhile, the same marker (SOD) was significantly lower in the IVM group compared to the control group.
Our findings showed that the level of testicular MDA (an index of oxidative process) was significantly improved in the IVM group and significantly lower in ZnNPs group compared to the control group. Meanwhile, rabbits in ZnNPs + IVM group showed no significant difference in MDA levels compared to the control group.
Histopathologic findings
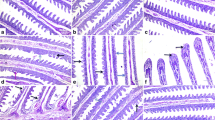
As shown in Fig. 1, testicular tissues of rabbits from the CTR and ZnNPs group showed normal histoarchitecture of seminiferous tubules, interstitial tissues, spermatogenic cells, Sertoli cells, and Leydig cells (Fig. 1A, D). In contrast, those that received IVM (IVM group) showed abnormal arrangement, degeneration, and vacuolization of spermatogenic cells. Moreover, necrosis of the epithelial cell lining the seminiferous tubules and multifocal separation of basement membrane were observed. Furthermore, necrotic spermatocytes and/or eosinophilic proteinaceous materials were identified with cellular debris occupying the central regions of the tubular laminae (Fig. 1B) and characteristic formation of a high number of sperm giant cells (Fig. 1C). Moreover, Sertoli cells were apparently reduced, whereas the intertubular tissues were widened and contained sparse distribution of Leydig cells with congested intertubular blood vessels. These lesions were ameliorated after inorganic Zn replacement through Zn nanoparticles in ZnNPs + IVM group (Fig. 1E, F).
Representative photomicrograph of testicular tissues of rabbits from control (A), IVM (B, C), ZnNPs (D), and ZnNPs + IVM (E, F) showed normal histoarchitecture of seminiferous tubules, interstitial tissues, spermatogenic cells, Sertoli cells, and Leydig cells (A, D). Abnormal arrangement and vacuolization of spermatogenic cells, eosinophilic proteinaceous materials (black arrow) and necrotic debris (blue arrow) occupying the central regions of the tubular laminae (B), formation of sperm giant cells (arrows) (C), and mild to moderate vacuolization and degeneration of spermatogenic cells and intertubular congestion (arrow) (E, F). H&E, scale bar = 50 µm (A, C, D, F), scale bar = 200 µm (B, E)
Epididymal tissues showed normal histologic limits of the epididymal tubules in the CTR and ZnNPs groups (Fig. 2A, C). Meanwhile, tissues from the IVM group showed necrotic eosinophilic proteinaceous materials and cellular debris occupying the central regions of the tubular laminae, degeneration and necrosis of epididymal tubule epithelial cells, and interstitial tissue fibrosis (Fig. 2B). Conversely, tissues from ZnNPs + IVM group exhibited amelioration of the most of these lesions. Interstitial tissue fibrosis and intertubular capillary congestion were evident in this group (Fig. 2D). The scoring of spermatogenesis was significantly (P < 0.05) declined in IVM group compared to control rats. However, oral administration of ZnNPs in ZnNPs + IVM group significantly (P < 0.05) improved the score of spermatogenesis compared to rats in IVM group (Fig. 3).
Representative photomicrograph of epididymal tissues of rabbits from control (A), IVM (B), ZnNPs (C), and ZnNPs + IVM (D) showed normal histologic limits of epididymal tubules (A, C), necrotic eosinophilic proteinaceous materials (black arrow) and cellular debris (blue arrow) occupying the central regions of the tubular laminae, degeneration and necrosis in epithelial lining (red arrow) (B), and amelioration of the most of these lesions with interstitial fibrosis and congestion of intertubular capillaries (arrow) (D). H&E, scale bar = 50 µm (A, C, D), scale bar = 200 µm (B)
Ameliorating effect of oral administration of ZnNPs (60 mg/kg diet) on spermatogenesis score of male rats intoxicated with IVM (0.2 mg/kg bwt) for 5 weeks. Data are expressed as the mean ± SEM. Different letters are significant at P < 0.05 with respect to the control group as a negative control (ANOVA with Dunnett’s multiple comparison test)
Immunohistochemical findings
Apoptotic activity of testicular spermatogenic cells and columnar epithelium of epididymal tubules were evaluated via immunohistochemical localization of caspase-3 (Figs. 4 and 5). In the testes, the immunoreactivity of caspase-3 was greatest in the IVM group followed by ZnNPs + IVM compared to CTR and ZnNPs groups. The highest apoptotic activity of the testicular cells was detected in the IVM group. In the epididymis, the immune staining of caspase-3 was significantly greatest in the IVM group followed by ZnNPs + IVM group compared to the CTR and ZnNPs groups. Figure 7 shows the non-parametric analysis for the mean count of immunological positive cells in the testes and epididymis.
Representative photomicrograph of testicular tissues of rabbits from control (A), IVM (B), ZnNPs (C), and ZnNPs + IVM (D) (sections stained with anti-PCNA immune staining; scale bar = 50 μm) showed strong (A, C) and mild to moderate immunoreactivity of anti-PCNA in lining epithelium of epididymis and strong immunoreactivity of intraluminal debris and interstitium (B)
On the contrary, as shown in Figs. 5 and 6, the proliferative activity of testicular and epididymal cells in the experimental groups was assessed using immunohistochemical localization of the proliferating cellular nuclear antigen (PCNA). In the testes (Fig. 6), the immunoreactivity of PCNA was mildly lower in the IVM and ZnNPs + IVM groups compared to the control groups (CTR and ZnNPs). Meanwhile, in the epididymal tubules, the proliferative activity was significantly decreased in the lining epithelium and increased in intraluminal tissue debris (Fig. 7). Figure 8 shows the non-parametric analysis for the mean count of immunological positive cells in testes and epididymis.
Representative photomicrograph of testicular tissues of rabbits from control (A), IVM (B), ZnNPs (C), and ZnNPs + IVM (D) (sections stained with anti-caspase-3 immune staining; scale bar = 50 μm) showed strong (B), moderate (D), and mild to negative immunoreactivity of anti-caspase-3 in testicular spermatogenic cells
Representative photomicrograph of testicular tissues of rabbits from control (A), IVM (B), ZnNPs (C), and ZnNPs + IVM (D) (sections stained with anti-caspase-3 immune staining; scale bar = 50 μm) showed strong (B), moderate (D), and negative immunoreactivity of anti-caspase-3 in lining epithelium of epididymis and strong immunoreactivity of intraluminal debris and interstitium (B, D)
Histomorphometric analysis of immune expression of a caspase-3 and b PCNA in testicular and epididymal tissues of rabbits; all scores were subjected to non-parametric analysis using Kruskal–Wallis test to assess the significance between mean scores obtained from Wilcoxon rank-sum test (P > chi-square < 0.05)
Discussion
The present study found that the ZnNPs group that received nano-Zn replacement had the highest live BW, total body gain, and total feed intake and the best average feed conversion ratio compared to the control group that received inorganic Zn (CTR). Our results are consistent with those presented in Tag-El Din (2019), which showed that nano-Zn supplementation at 60 mg/kg improved the final BW, gain, FI, and FCR in growing rabbits. Consistent with this data, Hassan et al. (2017) reported that rabbits fed a diet supplemented with nano-Zn (30 and 60 mg/kg) had increased BW, gain, and FCR compared to the control group. Other reports revealed that the dietary supplementation of 60 mg/kg of nano-Zn/kg promoted the highest BW gain and better feed conversion ratio than the control in broilers (Zhao et al. 2014) or dual-purpose chickens (Siddhartha et al. 2016). These results can be explained by the smaller size of nano-Zn, which allows for easier and faster passage through the cell membrane, thereby increasing its bioavailability and efficiency in promoting proper physiological functions, including DNA and protein syntheses, leading to better growth (Case and Carlson 2002; Onuegbu et al. 2018). Zn also participates in several enzymatic and metabolic functions, including carbohydrate, lipid, and protein metabolism (MacDonald 2000; Prasad and Kucuk 2002). Moreover, Zn is essential for free radical scavenging, immune system enhancement, and protection of the pancreatic tissue against oxidative damage, thereby ensuring optimal pancreatic function in secreting the digestive enzymes and subsequently improving nutrient digestibility (Zhao et al. 2014; Saleh et al. 2018).
On the contrary, our findings revealed that weekly IVM injections significantly deteriorated the growth and feed intake among rabbits receiving dietary inorganic Zn. Similarly, El-Shobokshy et al. (2022) found that repeated injection of IVM in female rabbits caused a decrease in BW, TBWG, and TFI. Similar to our findings, Khaldoun-Oularbi et al. (2015) and Khaldoun Oularbi et al. (2017) studied the adverse effects of emamectin benzoate in rats and concluded that the final BW and BWG decreased significantly due to a significant decrease in FI caused by the loss of appetite with decreasing in gastrointestinal tract nutrient absorption and deterioration of food conversion efficiency (Ball and Chhabra 1981; Sheriff et al. 2002). Our results also agree with those of Chahrazed et al. (2020) who observed that the repeated injection of high doses of IVM (2 mg/kg BW subcutaneously, 3 doses per week) for three consecutive weeks in rabbits significantly (P < 0.05) decreased the TFI due to the decreased in appetite, which significantly decreased the BW and total gain. Fortunately, the present study revealed that replacement of inorganic Zn with nano-Zn could overcome the negative effects of repeated IVM injections on the growth and FI. Hence, the co-administration of nano-Zn had ameliorative effects by reversing the negative effects of IVM on the BW, gain, and feed consumption in rabbits.
The present study revealed that nano-Zn significantly enhanced the dressing percentage and had no significant effect on the relative weights of the skin, head, and spleen. Meanwhile, rabbits receiving nano-Zn had significantly lower (P < 0.0001) relative weights of the heart, lungs, kidneys, and livers compared to those receiving inorganic Zn (CTR). Consistent with our results, Tag-El Din (2019) found that supplementation with nano-Zn at 60 mg/kg non-significantly decreased the relative weight of the head; however, they found that nano-Zn had no significant effect on the kidney, liver, and heart percentage. Similar to our findings, El-Katcha et al. (2017) reported that nano-Zn (45 and 60 mg/kg) improved the dressing percentage, lowered the relative weight of the liver, and had no significant effect on the relative weight of the spleen in broiler chickens compared to dietary inorganic Zn. Moreover, Lina et al. (2009) stated that nano-Zn significantly elevated the dressing percentage of boiler chickens.
Our data clarified that repeated IVM injections caused the worst dressing percentage and organ relative weights, whereas dietary nano-Zn (P < 0.0001) improved the dressing percentage and reversed the negative effects of IVM injection on organ relative weights, especially of the spleen and liver. Similarly, previous studies by Khaldoun-Oularbi et al. (2013), El Zoghby et al. (2015), and Khaldoun Oularbi et al. (2017) revealed that IVM significantly increased rat liver weight, whereas IVM co-treatment with, for instance, vitamin C decreased the absolute and relative weight of the liver. Moreover, Chahrazed et al. (2020) found that repeated high-dose injections of IVM in rabbits increased the relative weight of the liver and decreased lung weight due to IVM accumulation in lung tissue and generation of oxidative stress due to continuous IVM injection (Al-Jassim et al. 2016), whereas vitamin C reversed the aforementioned changes and protected the weights of the internal organs from the negative effects of IVM hazards.
Testosterone is a steroid hormone from the androgen group in mammals, reptiles, birds, and other vertebrates. In mammals, testosterone is primarily secreted in the testicles of males and the ovaries of females, although small amounts are also secreted by the adrenal glands (Vodo et al. 2013; El-Far 2013). Free testosterone, the serum testosterone not bound to sex hormone-binding globulin or albumin, is biologically active and able to exert its effects by permeating into cells and activating its receptor (Kevin et al. 2012; El-Far 2013). From the obtained data, we can clearly observe a significant increase in serum testosterone in the ZnNPs group at 16 weeks old, which may have been due to the role Zn plays in several biochemical processes and physiological functions. Reports have shown that Zn is required for the normal function of numerous structural proteins, enzymes, and hormones necessary for growth and development (Bao et al. 2009; Abdel-Wareth et al. 2020). The improved concentrations of testosterone in response to nano-Zn replacement might be due to the increased number of Leydig cells in the testis, which increases testosterone production (El-Masry et al. 1994; Imam et al. 2009; Abdel-Wareth et al. 2020). At 18 weeks old, no significant difference in testosterone concentration was observed between the ZnNPs and ZnNPs + IVM groups, with both groups reaching puberty at the same age and earlier than the other groups. These results highlight the vital role of ZnNPs as a powerful antioxidant that stimulates the process of steroid genesis and release of GnRH hormones from the anterior pituitary gland. Moreover, Zn acts as a scavenger for excessive superoxide radicals, thereby exhibiting antioxidant-like activities (Gavella and Lipovac 1988; Baiomy et al 2018). Chia et al. (2000) suggested that Zn may bind with free radicals in the seminal plasma, produced by abnormal spermatozoa, thereby decreasing the concentration of this element. Zn deficiency causes a lowering in testosterone levels (Hadwan et al. 2012; Chia et al. 2000).
In line with this, the present study revealed that dietary nano-Zn replacement significantly enhanced Zn concentrations in the testes of ZnNP-treated group. Our results were consistent with those obtained by Zhao et al. (2014) who found that serum Zn concentrations were significantly higher in broilers receiving 60 or 100 mg/kg nano-Zn compared to those receiving inorganic Zn. Moreover, Hassan et al. (2017) found that nano-Zn supplemented groups had significantly greater hepatic and serum Zn contents (P < 0.001) compared to the inorganic Zn groups. Moreover, similar findings in Japanese quails were reported by Reda et al. (2020). Given the increased concentration of Zn in ZnNPs group and its antioxidant role, it was unsurprising that rabbits treated with ZnNPs showed the best oxidative status. Zn is a fundamental component in SOD and is involved in the cellular scavenging of free radicals and reactive oxygen species (Prasad 2008; Abd El-Hack et al. 2018). MDA is an important index for lipid peroxidation and oxidative damage caused by reactive oxygen species (Bin-Jumah et al. 2020; El-Far et al. 2020). Our results revealed that GSH, SOD, and CAT activities had significantly increased in the testes of the ZnNPs group, whereas MDA concentrations had decreased. The depressed MDA levels in our study resulted from the suppressive effects of dietary nano-Zn replacement on reactive oxygen species generation, thereby decreasing MDA levels. These results are consisted with that reported by Reda et al. (2021) who found that dietary nano-Zn level significantly (P < 0.001) enhanced serum SOD and glutathione peroxidase (GPX) but reduced MDA levels. Moreover, Kamel et al. (2020) stated that the addition of nano-Zn into the diets of rabbits significantly increased glutathione and superoxide dismutase activities and decreased MDA levels. Moreover, Tag-El Din (2019) reported that plasma CAT content was slightly elevated following high-dose nano-Zn supplementation (60 mg/kg) in the diet of growing rabbits.
In contrast, rabbits in the IVM groups showed lower enzymatic activity of GSH, SOD, and CAT and higher MDA levels. This result highlights the harmful and immunosuppressive effects of IVM injection in rabbits. Omshi et al. (2018) stated that repeated IVM administration was associated with oxidative degradation in male rats. GabAllh et al. (2017) and Al-Jassim et al. (2015) also reported that IVM injection adversely affects the immune status of rabbits. However, the present study showed that IVM injection slightly affected the nano-Zn supplemented group. This result suggests the potential role of nano-Zn supplementation in alleviating the stressful effects of IVM injection. Such findings could be attributed to the enhanced Zn concentration resulting from nano-Zn supplementation, which plays a vital role in inhibiting seminal oxidative stress (Marzec-Wróblewska et al. 2012). These results are in agreement with those of Kamel et al. (2020) who reported that dietary nano-Zn supplementation improved the immune status of heat-stressed rabbits. Moreover, Saleh et al. (2018) reported similar findings in heat-stressed birds.
The effect of IVM on genital organ dimensions was demonstrated in the IVM group, especially with regard to the size and weight of the testis plus epididymis and weight of accessory glands. This result was in agreement with that reported by El-Far (2013) who demonstrated that therapeutic and double therapeutic doses of IVM in male rats significantly decreased testes size. The mentioned study attributed these changes to the degenerative changes observed in double therapeutic dose of IVM associated with complete necrosis and complete absence of spermatogenesis in most seminiferous tubules. The epididymis showed degeneration and necrosis in the epithelial cells of the epididymal tubules. These results are supported by our histopathological findings, which revealed severe testicular damages in the form of abnormal spermatogenic cell arrangement, focal degenerative and necrotic changes, and cellular debris occupying the tubular lumen in groups treated with IVM. Similar results have been previously reported by several authors (Elzoghby et al. 2015; Ahmed et al. 2020). Similarly, GabAllh et al. (2017) noticed degeneration and vacuolation of spermatogenic cells at therapeutic doses of IVM after 4 and 8 weeks. These lesions were associated with necrosis of the epithelial cells lining the seminiferous tubules with pyknotic nuclei and formation of a few sperm giant cells at a therapeutic dose after 8 weeks. Meanwhile, the same degenerative changes were also observed at double therapeutic dose wherein complete necrosis and complete absence of spermatogenesis in most of seminiferous tubules with high number of sperm giant cells were noted. In the current study, immunohistochemical staining with PCNA revealed a reduction in testicular tissue immune expression among groups treated with IVM, with PCNA playing a pivotal role in the S-phase of the cell cycle via DNA synthesis and replication (Strzalka and Ziemienowicz 2011). Therefore, the reduction in PCNA expression may promote suppression of DNA synthesis with subsequent DNA damage and reduced cellularity and spermatogenesis (Moshari et al. 2017). Similar results were obtained by Ahmed et al. (2020). In the current study, the immunocytochemical staining of caspase-3 revealed strong immunoreactivity in testicular tissues of IVM-exposed rabbits. Meanwhile, a significant reduction in the number of caspase-3 immune positive cells was observed in rabbits with a ZnNP-supplemented diet. The excessive reactive oxygen species production from the mitochondria of testicular cells could potentially interfere with the proper release of cytochrome c, which subsequently activates caspase-3 and induces cell apoptosis (Simon et al. 2000; Chung et al. 2003). IVM had been reported to collapse the mitochondrial membrane, leading to cell death and activation of various apoptotic markers, including caspase-3 (Khafaga and El-Sayed 2018). Similar results were previously obtained by Ahmed et al. (2020).
IVM affected semen parameters, such that the IVM group had significantly lower semen motility, livability, and concentration than the other groups. This result was in agreement with those reported by Elzoghby et al. (2015) who found that therapeutic and double therapeutic doses of IVM in male rats significantly decreased total sperm count and mortality. The accumulation of free radicals has been associated with a significant decrease in sperm motility and sperm plasma membrane integrity and a significant increase in sperm abnormality and DNA damage, leading to infertility (Potts et al. 2000). ZnNP-treated group had significantly better semen parameters than the other groups given that Zn plays a pivotal role in sperm cell function, including lipid flexibility, cell membrane stabilization (Chia et al. 2000), sperm capacitation, and acrosomal reaction (Eggert-Kruse et al. 2002). Moreover, reports had shown that Zn increased semen volume, total live sperm count, sperm motility, and conception in heat-stressed rabbits (El-Masry et al. 1994). Our findings clearly showed that ZnNPs + IVM group had better semen parameters than the IVM group, indicating the vital role of Zn in over 200 proteins and enzymes essential for male fertility (Kumar et al. 2006). Research has shown that Zn supplementation enhances the physical characteristics of semen, including ejaculate volume, sperm count, motility, seminal plasma antioxidants, and fertility rate (Amen and Muhammad 2016; Rahman et al. 2014; El-Speiy and El-Hanoun 2013; Rafique et al. 2010; Ghasemi et al. 2009; Oliveira et al. 2004; Maldjian et al. 1998). Rats treated with Zn showed an increase in sperm count, sperm motility, and testosterone levels, as well as improved testicular structure and spermatogenesis abnormalities caused by obesity (Ma et al. 2020). However, other studies have suggested no significant association between Zn and sperm quality (Eggert-Kruse et al. 2002; Lin et al. 2000).
Conclusion
Replacement of inorganic Zn with Zn nanoparticles enhanced the BW, weight gain, and FCR of male rabbits. In addition, fertility and oxidative parameters, as well as histopathologic findings, were also improved in ZnNP-treated rabbits. Dietary supplementation of ZnNPs for IVM-intoxicated rabbit ameliorated the negative impact of IVM and improved the performance of male rabbits potentially via its antioxidant and antiapoptotic pathways. Hence, we recommend including ZnNPs in the diets of rabbit exposed to IVM injections.
Data availability
The data used to support the findings of this study are included within the article and the coding of the data is available from the corresponding author upon reasonable request.
References
Abd El-Hack ME, Alagawany M, Arif M, Chaudhry MT, Emam M, Patra A (2017) Organic or inorganic zinc in poultry nutrition: a review. Worlds Poult Sci J 73(4):904–915
Abd El-Hack ME, Alagawany M, Amer SA, Arif M, Wahdan KM, El-Kholy MS (2018) Effect of dietary supplementation of organic zinc on laying performance, egg quality and some biochemical parameters of laying hens. J Anim Physiol Anim Nutr 102(2):542–554
Abdel-Wareth AAA, Kehraus S, Ali AHH, Ismail ZSH, Südekum KH (2015) Effects of temporary intensive feed restriction on performance, nutrient digestibility and carcass criteria of growing male Californian rabbits. Archiv Anim Nutr 69:69–78. https://doi.org/10.1080/1745039X.2014.1002672
Abdel-Wareth AA, Al-Kahtani MA, Alsyaad KM, Shalaby FM, Saadeldin IM, Alshammari FA, Mobashar M, Suleiman MH, Ali AH, Taqi MO, El-Sayed HG (2020) Combined supplementation of nano-zinc oxide and thyme oil improves the nutrient digestibility and reproductive fertility in the male Californian rabbits. Animals 10(12):2234. https://doi.org/10.3390/ani10122234
Ahmed AE, Alshehri A, Al-Kahtani MA, Elbehairi SI, Alshehri MA, Shati AA, Alfaifi MY, Al-Doais AA, Taha R, Morsy K, El-Mansi AA (2020) Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar albino rats. Biomed Pharmacother 124:109841
Al-Jassim AB, Jawad HA, Al-asoudi E, Jeedkhadim S (2015) Biochemical and histological alterations in the liver due to repeated administration of ivermectin alone or with combination of vitamin c in local female rabbits. J Int Acad Res Multidis 3:349–364
Al-Jassim KB, Jawad AADH, Al-Masoudi EA, Majeed SK (2016) Histopathological and biochemical effects of ivermectin on kidney functions, lung and the ameliorative effects of vitamin c in rabbits (Lupus cuniculus). Basrah J Vet Res 14:110–124
Al-Sagheer AA, Daader AH, Gabr HA, AbdEl-Moniem EA (2017) Palliative effects of extra virgin olive oil, gallic acid, and lemongrass oil dietary supplementation on growth performance, digestibility, carcass traits, and antioxidant status of heat-stressed growing New Zealand white rabbits. Environ Sci Pollut Res 24:6807–6818. https://doi.org/10.1007/s11356-017-8396-8
Amen MHM, Muhammad SS (2016) Effect of zinc supplementation on some physiological and growth traits in local male rabbit. World Vet J 6(3):151–155
Azad SK, Shariatmadari F, Torshizi MK, Ahmadi H (2017) Effect of zinc concentration and source on performance, tissue mineral status, activity of superoxide dismutase enzyme and lipid peroxidation of meat in broiler chickens. Anim Prod Sci 58(10):1837–1846
Azazi I, Gadelrab S, Elkomy H, Ahmed A (2018) Growth performance and feed utilization of growing rabbits fed diets containing olive cake meal supplemented with or without citric acid. Egypt J Rabbit Sci 28(2):241–262
Baiomy A, Hossam H, Emam K (2018) Effect of zinc oxide levels supplementation on semen characteristics and fertility rate of bucks rabbits under subtropical conditions. Egypt J Rabbit Sci 28(2):395–406
Ball LM, Chhabra RS (1981) Intestinal absorption of nutrients in rats treated with 2,3,7,8-tetra-chloro-dibenz-p-dioxin (TCDD). J Toxicol Environ Health 8:629–636
Bancroft JD (Eds.) (2013) Theory and practice of histological techniques, 7th ed. Churchill Livingstone, Edinburgh, New York, pp. 179–220.
Bao YM, Choct M, Iji PA, Bruerton K (2009) Optimal dietary inclusion of organically complexed zinc for broiler chickens. Br Poult Sci 50:95–102
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bin-Jumah, May, Mohamed E. Abd El-Hack, Sameh A. Abdelnour, Yasmeen A. Hendy, Hager A. Ghanem, Sara A. Alsafy, Asmaa F. Khafaga et al. Potential use of chromium to combat thermal stress in animals: a review. Sci Total Environ 707 (2020): 135996.
Case CL, Carlson MS (2002) Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J Anim Sci 80(7):1917–1924
Chahrazed M, Hassina K, Soumya B, Dalila T, Asma B, Meriem B, Nacira DZ (2020) Beneficial effects of ascorbic acid on ivermectin repeated high-dose therapy in rabbits: biochemical and histopathological investigations. Eur J Biol Res 11:1–13
Chasapis CT, Ntoupa PSA, Spiliopoulou CA, Stefanidou ME (2020) Recent aspects of the effects of zinc on human health. Arch Toxicol 94(5):1443–1460
Chia SE, Ong CN, Chua LH, Ho LM, Tay SK (2000) Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J Androl 21:53–57
Chung YM, Bae YS, Lee SY (2003) Molecular ordering of ROS production, mitochondrial changes, and caspase activation during sodium salicylate-induced apoptosis. Free Radic Biol Med 34(4):434–442
Dosoky, Waleed M., Aya A. Al-Banna, Soliman M. Zahran, Soha A. Farag, Nader R. Abdelsalam, and Asmaa F. Khafaga (2022) Zinc oxide nanoparticles induce dose-dependent toxicosis in broiler chickens reared in summer season. Environ Sci Pollut Res :1–20.
Eggert-Kruse W, Zwick EM, Batschulat K, Rohr G, Armbruster FP, Petzoldt D, Strowitzki T (2002) Are zinc levels in seminal plasma associated with seminal leukocytes and other determinants of semen quality? Fertil Steril 77:260–269
El Zoghby RR, Amin A, Hamouda AF, Ali AF (2015) Toxicological and pathological studies of ivermectin on male albino rats. J Am Sci 11(3):73–83
El-Ashram S, Aboelhadid SM, Abdel-Kafy ESM, Hashem SA, Mahrous LN, Farghly EM, Kamel AA (2020) Investigation of pre-and post-weaning mortalities in rabbits bred in Egypt, with reference to parasitic and bacterial causes. Animals 10(3):537
El-Far AH (2013) Effect of therapeutic and double therapeutic doses of ivermectin on oxidative status and reproductive hormones in male rabbits. Am J Anim Vet Sci 8(3):128–133
El-Far AH, Lebda MA, Noreldin AE, Atta MS, Elewa YH, Elfeky M, Mousa SA (2020) Quercetin attenuates pancreatic and renal D-galactose-induced aging-related oxidative alterations in rats. Int J Mol Sci 21(12):4348
El-Katcha M, Soltan MA, El-badry M (2017) Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alex J Vet Sci 55(2):129–145
El-Masry KA, Nasr AS, Kamal TH (1994) Influences of season and dietary supplementation with selenium and vitamin E or Zinc on some blood constituents and semen quality of New Zealand white rabbit males. World Rabbit Sci 2,79e86
El-Shobokshy SA, Abo-Samaha MI, Abd El-Rheem SM, Sahwan FM, Wirtu G, Soltan MAK, Emam M (2022) Dietary supplementation of nano-selenium eliminates the negative effects of long-term ivermectin injection on growth and reproductive performance of female rabbits. J Adv Vet Anim Res 9(1):128–137
El-Speiy ME, El-Hanoun AM (2013) Effect of queracetin (onion juice) and zinc sulphate on reproductive performance of male rabbits under hot summer conditions. Egypt Poult Sci 33(2):331–347
Elzoghby RR, Amin A, Hamouda AF, Ali AF (2015) Toxicological and pathological studies of ivermectin on male albino rats. Jam Sci 11:73–83
Fairweather-Tait SJ, de Sesmaisons A (2019) Approaches used to estimate bioavailability when deriving dietary reference values for iron and zinc in adults. Proc Nutr Soc 78(1):27–33
GabAllh M, El-Mashad AE, Amin AA, Darweish MM (2017) Pathological studies on effects of ivermectin on male and female rabbits. J BenhaVet.Med 32:104–112.
Gavella M, Lipovac V (1988) In vitro effect of zinc on oxidative changes in human semen. Andrologia 30:317–323
Ghasemi N, Babaei H, Azizallahi S, Kheradmand A (2009) Effect of long-term administration of zinc after scrotal heating on mice spermatozoa and subsequent offspring quality. Andrologia 41:222–228
Hadwan MH, Almashhedy LA, Alsalman AR (2012) Oral zinc supplementation restore high molecular weight seminal zinc binding protein to normal value in Iraqi infertile men. BMC Urol 12:32
Hassan E, El-Neweshy M, Hassan M, Noreldin A (2019) Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: possible mechanisms are involved. Life Sci 230:132–140
Hassan FAM, Mahmoud R, El-Araby IE (2017). Growth performance, serum biochemical, economic evaluation and IL6 gene expression in growing rabbits fed diets supplemented with zinc nanoparticles. Zagazig Vet J 45 (3),238–249. https://zvjz.journals.ekb.eg/article_7949.html
Imam S, Ansari MR, Kumar R, Mudga V, Varshney VP, Dass RS (2009) Effect of inorganic and organic zinc supplementation on serum testosterone level in murrah buffalo (Bubalus bubalis) bulls. Ind J Anim Sci 79:61
Johnsen SG (1970) Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Horm Res Paediatr 1:2–25
Johnson-Arbor K (2022) Ivermectin: a mini-review. Clin Toxicol 60(5):571–575
Joshi G, Quadir SS, Yadav KS (2021) Road map to the treatment of neglected tropical diseases: nanocarriers interventions. J Control Release 339:51–74
Kamel D, Abdel-Khalek A, Gabr S (2020) Effect of dietary zinc-oxide or nano-zinc oxide on growth performance, oxidative stress, and immunity of growing rabbits under hot climate conditions. J Anim Poult Prod 11:565–571
Kevin M, Pantalone DO, Ecnu CCD (2012) Male hypogonadism: more than just a low testosterone. Cleveland Clin J Med 79:717–725. https://doi.org/10.3949/ccjm.79a.11174
Khafaga AF, El-Sayed YS (2018) All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: in vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 downexpression. Naunyn-Schmiedeberg’s Archives of Pharmacology 391(1):59–70
Khaldoun Oularbi H, Richeval C, Lebaili N, Zerrouki-Daoudi N, Baha M, Djennas N, Allorge D (2017) Ameliorative effect of vitamin C against hepatotoxicity induced by emamectin benzoate in rats. Human Exp Toxicol 36(7):709–717
Khaldoun-Oularbi H, Richeval C, Djenas N, Lhermitte M, Humbert L, Baz A (2013) Effect of sub-acute exposure to abamectin “insecticide” on liver rats (Rattus norvegicus). Ann Toxicol Anal 25(2):63–70
Khaldoun-Oularbi H, Allorge D, Richeval C, Lhermitte M, Djenas N (2015) Emamectin benzoate (Proclaim®) mediates biochemical changes and histopathological damage in the kidney of male Wistar rats (Rattus norvegicus). Toxicol Anal Clin 27(2):72–80
Khan Sharun TS, Aneesha VA, Dhama K, Pawde AM, Pal A (2019) Current therapeutic applications and pharmacokinetic modulations of ivermectin. Vet World 12(8):1204
Kumar N, Verma RP, Singh LP, Varshney VP, Dass RS (2006) Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus × Bos taurus) bulls. Reprod Nutr Dev 46:663–675
Lin YC, Chang TC, Tseng YJ, Lin YL, Huang FJ, Kung FT, Chang SY (2000) Seminal plasma zinc levels and sperm motion characteristics in infertile samples. Chang Gung Med J 23:260–266
Lina T, Jianyang J, Fenghua Z, Huiying R, Wenli L (2009) Effects of nano-zinc oxide on the production and dressing performance of broiler. Chinese Agri Sci Bul 2:318
Ma J, Han R, Li Y, Cui T, Wang S (2020) The mechanism of zinc sulfate in improving fertility in obese rats analyzed by sperm proteomic analysis. Biomed Res Int 2020.https://doi.org/10.1155/2020/9876363
Macdonald RS (2000) The role of zinc in growth and cell proliferation. Nutr J 130:1500–1508
Maldjian A, Cerolini S, Surai P, Speake BK (1998) The effect of vitamin E, green tea extracts and catechin on the in vitro storage of turkey spermatozoa at room temperature. Avian Poult Biol Rev 9(4):143–151
Mangoli E, Talebi AR, Anvari M, Pourentezari M (2013) Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med 11(1):53–60
Marzec-Wróblewska U, Kamiński P, Łakota P (2012) Influence of chemical elements on mammalian spermatozoa. Folia Biol (praha) 58:7–15
Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Diaz LE, Marcos A (2013) Zinc: dietary intake and impact of supplementation on immune function in elderly. Age 35(3):839–860
Moshari S, Nejati V, Najafi G (2017) Nanomicelle curcumin-induced DNA fragmentation in testicular tissue; correlation between mitochondria dependent apoptosis and failed PCNA-related hemostasis. Acta Histochem 119(4):372–381
NRC (1977) Nutrient requirements of rabbits, 2nd, revised. National Academy Press, Washington, DC
Oliveira CEA, Badú CA, Ferreira WM, Kamwa EB, Lana AMQ (2004) Effects of dietary zinc supplementation on spermatic characteristics of rabbit breeders. Proceedings - 8th World Rabbit Congress –September 7–10. Puebla, Mexico.
Omshi HFS, Abbasalipourkabir R, Abbasalipourkabir M, Nabyan S, Bashiri A, Ghafourikhosroshahi A (2018) Effect of vitamin A and vitamin C on attenuation of ivermectin-induced toxicity in male Wistar rats. Environ Sci Pollut Res 25(29):29408–29417
Onuegbu C, Aggarwal A, Singh NB (2018) ZnO nanoparticles as feed supplement on growth performance of cultured African catfish fingerlings. J Sci Ind Res 77(4):213–218
PARA PA GS, Wakchaure R, Sharma R, Mahajan T, Praveen PK (2015) Rabbit meat has the potential of being a possible alternative to other meats as a protein source: a brief review. Int J Phar Biomedi Res 2:17-19
Potts RJ, Notarianni LJ, Jefferies TM (2000) Seminal plasma reduces exogenous oxidative damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res 44:249–256
Powell MD, Yousaf MN, Rasmussen KJ, Kollner B, Zou J, Secombes C, Speare DJ (2014) Immunohistochemical localization of inflammatory cells and cell cycle proteins in the gills of Loma salmonae infected rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 40:91–98
Prasad AS (2008) Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 43(5):370–377
Prasad AS, Kucuk O (2002) Zinc in cancer prevention. Cancer Metastasis Rev 21:291–295
Rafique M, Naqvi A, Nankani K (2010) Zinc improves the quality of semen lab rats. Med Chan 16:619–622
Rahman HU, Qureshi MS, Khan RU (2014) Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of Beetal bucks. Reprod Dom Anim 49:1004–1007
Reda FM, El-Saadony MT, Elnesr SS, Alagawany M, Tufarelli V (2020) Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals 10(5):754
Reda FM, El-Saadony MT, El-Rayes TK, Attia AI, El-Sayed SA, Ahmed SY, Madkour M, Alagawany M (2021) Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Italian J Anim Sci 20(1):324–335. https://doi.org/10.1080/1828051X.2021.1886001
Saleh AA, Ragab MM, Ahmed EAM, Abudabos AM, Ebeid TA (2018) Effect of dietary zinc-methionine supplementation on growth performance, nutrient utilization, antioxidative properties and immune response in broiler chickens under high ambient temperature. J Appl Anim Res 46:820–827
Saleh H, Nassar AM, Noreldin AE, Samak D, Elshony N, Wasef L, Elewa YH, Hassan SM, Saati AA, Hetta HF, Batiha GES (2020) Chemo-protective potential of cerium oxide nanoparticles against fipronil-induced oxidative stress, apoptosis, inflammation and reproductive dysfunction in male white albino rats. Molecules 25(15):3479
SAS (2014) Statistical Analysis System. SAS Institute Inc, Cary, NC, USA
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Sheriff JC, Kotze AC, Sangster NC (2002) Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology 125:477–484
Siddhartha S, Pathak S, Venkata RK, Prasoon S (2016) Influence of different sources of zinc on growth performance of dual purpose chicken. J Bio Innov 5(5):663–672
Simon H-U, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Strzalka W, Ziemienowicz A (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot 107:1127–1140. https://doi.org/10.1093/aob/mcq243
Swain PS, Rao SB, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2(3):134–141
Tag-El Din NTH (2019) Effects of dietary nano-zinc and nano-selenium on productive and physiological performance of growing rabbits at fattening period. Egypt J Nutr Feeds 22(1):79-89
Tietz N (1995) Clinical guide to laboratory tests. 3rd edition, Philadelphia, PA, WA Saunders Co, pp. 322.
Underwood E (2012) Trace elements in human and animal nutrition. Elsevier
Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM (2013) Testosterone-induced effects on lipids and inflammation. Mediators Inflammat 183041-183048.https://doi.org/10.1155/2013/183041
Zhao CY, Tan SX, Xiao XY, Qiu XS, Pan JQ, Tang ZX (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160(3):361–367
Acknowledgements
The authors gratefully acknowledge the Egyptian Knowledge Bank (EKB) for the support in language editing and in open access publication.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors contributed to the present study as follows: data curation: SAE, MIA, AFK; resources: FMS, SMAE, ME; formal analysis: MIA, AFK; software: SAE, FMS; writing—original draft: SMAE, ME; writing—review and editing: AFK, MIA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of University of Alexandria approved the experimental protocol used in this study (Permit #2021/013/97).
Consent for publication
All the authors agreed to the publication and current article does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Shobokshy, S.A., Abo-Samaha, M.I., Sahwan, F.M. et al. Implication of apoptosis and oxidative stress in mitigation of ivermectin long-term hazards by zinc nanoparticles in male rabbits. Environ Sci Pollut Res 30, 26982–26997 (2023). https://doi.org/10.1007/s11356-022-24095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24095-1