Abstract
The presence of high concentrations of arsenic species in drinking water and other water bodies has become one of the most critical environmental concerns. Therefore, decontamination of arsenic-containing water is essential for improved health and environmental concern. In recent years, nano-adsorbents have been widely used for the adsorptive removal of arsenic from water. Separating existing nano-adsorbents from treated waters, on the other hand, is a critical issue for their potential applications in natural water treatment. To address these issues and to effectively remove arsenic from water, researchers looked at iron oxide-based magnetic nanocomposite adsorbents. The magnetic nanoadsorbents have the benefit of surface functionalization, making it easier to target a specific pollutant for adsorption, and magnetic separation. In addition, magnetic nanoparticles have a large surface area, high chemical inertness, superparamagnetic, high magnetic susceptibility, small particle size, and large specific surface area, and are especially easily separated in a magnetic field. Magnetic nano-adsorbents have been discovered to have a lot of potential for eliminating arsenic from water. The recent advances in magnetic nano-absorbents for the cleanup of arsenic species from water are summarized in this paper. Future perspectives and directions were also discussed in this article. This will help budding researchers for the further advancement of magnetic nanocomposites for the treatment of water and wastewater contaminated with arsenic.


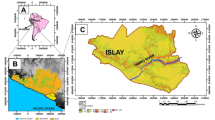
Reproduced with permission from Springer Nature

Reproduced with permission from Springer Nature

Reproduced with permission from Springer Nature

Reproduced with permission from Springer Nature


Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Adegoke HI, Adekola FA, Fatoki OS, Ximba BJ (2014) A comparative study of sorption of As (V) ions on nanoparticle hematite, goethite and magnetite. TechConnect Briefs 1:184–187
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290
Asta MP, Cama J, Martínez M (2009) Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J Hazard Mater 171:965–972
Augusto PA, Castelo-Grande T, Merchan L, Estevez AM, Quintero X, Barbosa D (2019) Landfill leachate treatment by sorption in magnetic particles: preliminary study. Sci Total Environ 648:636–668
Awasthi A, Jadhao P, Kumari K (2019) Clay nano-adsorbent: structures, applications and mechanism for water treatment. SN Appl Sci 1(9):1–21
Babu CM, Palanisamy B, Sundaravel B, Palanichamy M, Murugesan V (2013) A novel magnetic Fe3O4/SiO2 core-shell nanorods for the removal of arsenic. J Nanosci Nanotechnol 13(4):2517–2527
Babu CM, Vinodh R, Sundaravel B, Abidov A, Peng MM, Cha WS, Jang HT (2016) Characterization of reduced graphene oxide supported mesoporous Fe2O3/TiO2 nanoparticles and Adsorption of As(III) and As(V) from potable water. J Taiwan Inst Chem Eng 62:199–208
Baigorria E, Cano L, Sapag K, Alvarez V (2021) Removal efficiency of As(III) from aqueous solutions using natural and Fe(III) modified bentonites. Environ Technol. https://doi.org/10.1080/09593330.2021.1934559
Bangari RS, Singh AK, Namsani S, Singh JK, Sinha N (2019) Magnetite-coated boron nitride nanosheets for the removal of arsenic(V) from water. ACS Appl Mater Interfaces 11(21):19017–19028
Begum S, Ahmaruzzaman M (2018) Green synthesis of SnO2 quantum dots using Parkia speciosa Hassk pods extract for the evaluation of anti-oxidant and photocatalytic properties. J Photochem Photobiol, B 184:44–53
Bissen M, Frimmel FH (2003) Arsenic: a review—part I—: occurrence, toxicity, speciation, mobility. Acta Hydrochimica et Hydrobiologica 2003;31(1):9–18
Bhandari H, Garg S, Gaba R (2021) Advanced nanocomposites for removal of heavy metals from wastewater. Macromol Symp 397(1):2000337. https://doi.org/10.1002/masy.202000337
Bhattacharjee A, Ahmaruzzaman M (2015) A green approach for the synthesis of SnO2 nanoparticles and its application in the reduction of p-nitrophenol. Mater Lett 157:260–264
Bhaumik M, Noubactep C, Gupta VK, McCrindle RI, Maity A (2015) Polyaniline/Fe0 composite nanofibers: an excellent adsorbent for the removal of arsenic from aqueous solutions. Chem Eng J 271:135–146
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. II Kinetics. J Am Chem Soc 69:2836–2848
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35(3):647–654
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691
Bruanuer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 38:309–319
Cao Y, Li X (2014) Adsorption of graphene for the removal of inorganic pollutants in water purification: a review. Adsorption 20(5–6):713–727. https://doi.org/10.1007/s10450-014-9615-y
Chang Q, Lin W, Ying WC (2010) Preparation of iron-impregnated granular activated carbon for arsenic removal from drinking water. J Hazard Mater 184(1–3):515–522. https://doi.org/10.1016/j.jhazmat.2010.08.066
Chaudhary J, Thakur S, Sharma M, Gupta VK, Thakur VK (2020) Development of biodegradable agar-agar/gelatin-based superabsorbent hydrogel as an efficient moisture-retaining agent. Biomolecules 10:939. https://doi.org/10.3390/biom10060939
Chaudhry SA, Zaidi Z, Siddiqui SI (2017) Isotherm, kinetic and thermodynamics of arsenic adsorption onto iron-zirconium binary oxide-coated sand (IZBOCS): modelling and process optimization. J Mol Liq 229:230–240
Chowdhury T, Zhang L, Zhang J, Aggarwal S (2018) Removal of arsenic(iii) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 8(12):1062. https://doi.org/10.3390/nano8121062
de Oliveira HAL, Campos AFC, Gomide G, Zhang Y, Ghoshal S (2020) Elaboration of a core@shell bimagnetic nanoadsorbent (CoFe2O4@γ-Fe2O3) for the removal of As(V) from water. Colloids Surf, A 600(5):125002
Deliyanni EA, Bakoyannnnakis DN, Zouboulis AI, Matis KA (2003) Sorption of As(V) ions by akaganeite-type nanocrystals. Chemosphere 50:155–163
Deliyanni EA, Nalbandian LK, Matis A (2006) Adsorptive removal of arsenites by a nanocrystalline hybrid surfactant–akaganeite sorbent. J Colloid Interface Sci 302(2):458–466
Deng M, Wu X, Zhu A, Zhang Q, Liu Q (2019) Well-dispersed TiO2 nanoparticles anchored on Fe3O4 magnetic nanosheets for efficient arsenic removal. J Environ Manage 237:63–74
Doušová B, Grygar T, Martaus A, Fuitová L, Koloušek D, Machovicˇ V (2006) Sorption of As (V) on aluminosilicates treated with Fe (II) nanoparticles. J Colloid Interface Sci 302:424–431
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal Proceedings of the Academy of Sciences. Phys Chem Sect USSR 55:331–333
Dutta S, Manna K, Srivastava SK, Gupta AK, Yadav MK (2020) Hollow polyaniline Microsphere/Fe3O4 nanocomposite as an effective adsorbent for removal of arsenic from water. Sci Rep 10:4982
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of non-electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR. Otd Khim Nauk 2:209–216
Faria MCS, Rosemberg RS, Bomfeti CA, Monteiro DS, Barbosa F, Oliveira LC, Rodrigues JL (2014) Arsenic removal from contaminated water by ultrafine δ-FeOOH adsorbents. Chem Eng J 237:47–54
Farideh JB, Najafpoor AA, Davoudi M et al (2018) Environ Prog Sustain Energy 37:951–960
Feng L, Cao M, Ma X, Zhu Y, Hu C (2012) Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 217–218:439–446
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Gopiraman M, Soo Kim I (2019) Carbon nanocomposites: preparation and its application in catalytic organic transformations. Nanocomposites-Recent Evolutions. https://doi.org/10.5772/intechopen.81109
Hao L, Song H, Zhang L, Wan X, Tang Y, Lv Y (2012) SiO2/graphene composite for highly selective adsorption of Pb(ii) ion. J Colloid Interface Sci 369(1):381–387. https://doi.org/10.1016/j.jcis.2011.12.023
Hao L, Liu M, Wang N, Li G (2018) A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv 8:39545–39560
Hasanzadeh M, Farajbakhsh F, Shadjou N, Jouyban A (2015) Mesoporous (organo) silica decorated with magnetic nanoparticles as a reusable nanoadsorbent for arsenic removal from water samples. Environ Technol 36(1):36–44
Hernández-Flores H, Pariona N, Herrera-Trejo M, Hdz-García HM, Mtz-Enriquez AI (2018) Concrete/maghemite nanocomposites as novel adsorbents for arsenic removal. J Mol Struct 1171:9–16
Hu JS, Zhong LS, Song WG, Wan LJ (2008) Synthesis of hierarchically structured metal oxides and their application in heavy metal ion removal. Adv Mater 20(15):2977–2982
Hu Q, Liu Y, Gu X, Zhao Y (2017) Adsorption behavior and mechanism of different arsenic species on mesoporous MnFe2O4 magnetic nanoparticles. Chemosphere 181:328–336
Huang JG, Liu JC (1997) Enhanced removal of As(V) from water with iron-coated spent catalyst. Sep Sci Technol 32(9):1557–1569
Jamali-Behnam F, Najafpoor AA, Davoudi M, Rohani-Bastami T, Alidadi H, Esmaily H, Dolatabadi M (2018) Adsorptive removal of arsenic from aqueous solutions using magnetite nanoparticles and silica-coated magnetite nanoparticles. Environ Prog Sustain Energy 37(3):951–960
Joshi A, Chaudhuri M (1996) Removal of arsenic from groundwater by iron oxide-coated sand. J Environ Eng 122(8):769–771
Joshi S, Sharma M, Kumari A, Shrestha S, Shrestha B (2019) Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl Sci 9(18):3732
Kakihara Y, Fukunishi T, Takeda S, Nishijima S, Nakahira A (2004) Superconducting high gradient magnetic separation for purification of wastewater from paper factory. IEEE Trans Appl Supercond 14(2):1565–1567
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39(5):1291–1298
Kango S, Kumar R (2016) Magnetite nanoparticles coated sand for arsenic removal from drinking water. Environ Earth Sci 75(5):1–12
Karakaş ZK, Boncukcuoğlu R, Karakaş IH (2017) Adsorptive properties of As (III) from aqueous solution using magnetic nickel ferrite (NiFe2O4) nanoparticles: isotherm and kinetic studies. Sep Sci Technol 52(1):21–34
Karki B, Pandey P, Rajbhandari R, Joshi S, Koirala AR, Sharma RK, Pant HR (2019) Facile synthesis of magnetic activated carbon composite for arsenic adsorption. J Inst Eng 15(2):71–78. https://doi.org/10.3126/jie.v15i2.27643
Kotwicki V (1991) Water in the universe. Hydrol Sci J- Des Sciences Hydrologiques 36(1):49–66
Kumar ASK, Jiang SJ (2017) Synthesis of magnetically separable and recyclable magnetic nanoparticles decorated with β-cyclodextrin functionalized graphene oxide an excellent adsorption of As(V)/(III). J Mol Liq 237:387–401
Kumar S, Nair RR, Pillai PB, Gupta SN, Iyengar MAR, Sood AK (2014) Graphene oxide–MnFe2O4 magnetic nanohybrids for efficient removal of lead and arsenic from water. ACS Appl Mater Interfaces 6:17426–17436
Kwon HW, Shin TC, Kim JJ, Ha DW, Kim MG, Kim YH (2018) Removal of arsenic from aqueous phase using magnetized activated carbon and magnetic separation. Prog Supercond Cryogenics (PSAC) 20:1–5. https://doi.org/10.9714/psac.2018.20.2.001
Kyzas GZ, Matis KA (2016) Methods of arsenic wastes recycling: focus on flotation. J Mol Liq 214:37–45
Lackovic JA, Nikolaidis NP, Dobbs GM (2000) Inorganic arsenic removal by zero-valent iron. Environ Eng Sci 17(1):29–39
Lafferty BJ, Loeppert RH (2005) Methyl arsenic adsorption and desorption behavior on iron oxides. Environ Sci Technol 39:2120–2127
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids J Am Chem Soc 38:2221–2295
Lakshmipathiraj P, Narasimhan BRV, Prabhakar S, Bhaskar GR (2006) Adsorption of arsenate on synthetic goethite from aqueous solutions. J Hazard Mater 136:281–287
Leist M, Casey RJ, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76:125–138
Leist M, Casey RJ, Caridi D (2003) The fixation and leaching of cement stabilised arsenic. Waste Manage 23:353–359
Lin YF, Chen JL (2013) Synthesis of mesoporous maghemite (gamma-Fe2O3) nanostructures with enhanced arsenic removal efficiency. RSC Adv 3(35):15344–15349
Lin TF, Wu JK (2001) Adsorption of arsenite and arsenate within activated alumina grains: equilibrium and kinetics. Water Res 35(8):2049–2057
Lin YJ, Cao WZ, Ouyang T, Chen BY, Chang CY (2017) Developing sustainable graphene-doped titanium nanotube coated to superparamagnetic nanoparticles for arsenic recovery. J Taiwan Inst Chem Eng 70:3111–3318
Lin YJ, Cao WZ, Ouyang T, Mohan S, Chang CT (2018) Adsorption mechanism of magnetic nanoparticles doped with graphene oxide and titanium nanotubes for As(III) removal. Materialia 3:79–89
Liu Z, Zhang FS, Sasai R (2010) Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem Eng J 160(1):57–62
Liu CH, Chuang YH, Chen TY et al (2015) Environ Sci Technol 49:7726–7734
Liu J, Kong L, Huang X, Liu M, Li L (2018) Removal of arsenic(v) from aqueous solutions using sulfur-doped Fe3O4 nanoparticles. RSC Adv 8(71):40804–40812
Ma J, Zhu Z, Chen B, Yang M, Zhou H, Li C, Yu F, Chen J (2013) One-pot, large-scale synthesis of magnetic activated carbon nanotubes and their applications for arsenic removal. J Mater Chem A 1(15):4662. https://doi.org/10.1039/c3ta10329c
Magalhaes MCF (2002) Arsenic. An environmental problem limited by solubility. Pure Appl Chem 74(10):1843–1850
Maji SK, Kao YH, Liao PY, Lin YJ, Liu CW (2013) Implementation of the adsorbent iron-oxide-coated natural rock (IOCNR) on synthetic As(III) and on real arsenic-bearing sample with filter. Appl Surf Sci 284:40–48
Mamindy-Pajany Y, Hurel C, Marmier N, Roméo M (2009) Arsenic adsorption onto hematite and goethite. Comptes Rendus Chimie 12.https://doi.org/10.1016/j.crci.2008.10.012
Martinson CA, Reddy KJ (2009) Adsorption of arsenic (III) and arsenic (V) by cupric oxide nanoparticles. J Colloid Interface Sci 336(2):406–411
McKay CP (2014) Requirements and limits for life in the context of exoplanets. PNAS 111(35):12628–12633
Mirshahghassemi S, Ebner AD, Cai B, Lead JR (2017) Application of high gradient magnetic separation for oil remediation using polymer-coated magnetic nanoparticles. Sep Purif Technol 179:328–334
Mishra AK, Ramaprabhu S (2010) Magnetite decorated multiwalled carbon nanotube-based supercapacitor for arsenic removal and desalination of seawater. J Phys Chem C 114:2583–2590
Mishra PK, Gahlyan P, Kumar R, Rai PK (2018) Aero-gel based cerium doped iron oxide solid solution for ultrafast removal of arsenic. ACS Sustain Chem Eng 6:10668–10678
Mohammadi Nodeh MK, Gabris MA, Rashidi Nodeh H, Bidhendi ME (2018) Efficient removal of arsenic(III) from aqueous media using magnetic polyaniline-doped strontium–titanium nanocomposite. Environ Sci Pollut Res 25(17):16864–16874
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents: a critical review. J Hazard Mater 142:1–53
Mudzielwana R, Gitari MW, Ndungu P (2019) Uptake of As(V) from groundwater using Fe-Mn oxides modified kaolin clay: physicochemical characterization and adsorption data modeling. Water 11(6):1245. https://doi.org/10.3390/w11061245
Mukherjee A, Bhattacharya P, Shi F, Fryar AE, Mukherjee AB, Xie ZM, Jacks G, Bundschuh J (2009) Chemical evolution in the high arsenic groundwater of the Huhhot basin (Inner Mongolia, PR China) and its difference from the western Bengal basin (India). Appl Geochem 24(10):1835–1851
Nikić J, Watson MA, Isakovski MK, Tubić A, Šolić M, Kordić B, Agbaba J (2019) Synthesis, characterization and application of magnetic nanoparticles modified with Fe-Mn binary oxide for enhanced removal of As(III) and As(V). Environ Technol 42(16):2527–2539
Nishijima S, Takeda SI (2006) Superconducting high gradient magnetic separation for purification of wastewater from paper factory. IEEE Trans Appl Supercond 16(2):1142–1145
Ntim SA, Mitra S (2011) Removal of trace arsenic to meet drinking water standards using iron oxide coated multiwall carbon nanotubes. J Chem Eng Data 56(5):2077–2083
Önnby L, Pakade V, Mattiasson B, Kirsebom H (2012) Polymer composite adsorbents using particles of molecularly imprinted polymers or aluminium oxide nanoparticles for treatment of arsenic contaminated waters. Water Res 46(13):4111–4120
Ouni L, Ramazani A, Taghavi Fardood S (2019) An overview of carbon nanotubes role in heavy metals removal from wastewater. Front Chem Sci Eng 13(2):274–295. https://doi.org/10.1007/s11705-018-1765-0
Park WK, Yoon YJ, Kim S, Yoo S, Do Y, Kang JW, Yoon DH, Yang WS (2016) Feasible water flow filter with facilely functionalized Fe3O4-non-oxidative graphene/CNT composites for arsenic removal. J Environ Chem Eng 4:3246–3252
Paul B, Parashar V, Mishra A (2015) Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ Sci: Water Res Technol 1:77–83
Podder MS, Majumder CB (2015) SD/MnFe2O4 composite, a biosorbent for As(III) and As(V) removal from wastewater: optimization and isotherm study. J Mol Liq 212:382–404
Podgorski J, Berg M (2020) Global threat of arsenic in groundwater. Science 368(6493):845–850
Powell CD, Atkinson AJ, Ma Y, Marcos-Hernandez M, Villagran D, Westerhoff P, Wong MS (2020) Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J Nanopart Res 22:48
Predoi D, Iconaru SL, Predoi MV, Motelica-Heino M (2020) removal and oxidation of As(III) from water using iron oxide coated CTAB as adsorbent. Polymers 12(8):1687
Puri N, Gupta A, Mishra A (2021) Recent advances on nano-adsorbents and nanomembranes for the remediation of water. J Clean Prod 322:129051
Ramesha GK, Vijaya Kumara A, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361(1):270–277. https://doi.org/10.1016/j.jcis.2011.05.050
Ramos Guivar JA, Bustamante DA, Gonzalez JC, Sanches EA, Morales MA, Raez JM, López-Muñoz MJ, Arencibia A (2018) Adsorption of arsenite and arsenate on binary and ternary magnetic nanocomposites with high iron oxide content. Appl Surf Sci 454:87–100
Rashid M, Sterbinsky GE, Pinilla MÁG, Cai Y, O’Shea KE (2018) Kinetic and mechanistic evaluation of inorganic arsenic species adsorption onto humic acid grafted magnetite nanoparticles. J Phys Chem C 122(25):13540–13547
Raval NP, Kumar M (2021) Geogenic arsenic removal through core–shell-based functionalized nanoparticles: Groundwater in-situ treatment perspective in the post–COVID Anthropocene. J Hazard Mater 402:123466
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. John Wiley & Sons, West Sussex, UK
Ravenscroft P, Brammer H, Richards K (2011) Arsenic pollution: a global synthesis. John Wiley & Sons. ISBN: 978-1-405-18601-8
Redlich O, Peterson DI (1959) A useful adsorption isotherm. J Phys Chem 63:1024–1026
Sadegh H, Ali GA, Gupta VK, Makhlouf ASH, Shahryari-Ghoshekandi R, Nadagouda MN, Sillanpää M, Megiel E (2017) The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J Nanostruct Chem 7(1):1–14
Sahu UK, Sahu S, Mahapatra SS, Patel RK (2017) Cigarette soot activated carbon modified with Fe3O4 nanoparticles as an effective adsorbent for As(III) and As(V): material preparation, characterization and adsorption mechanism study. J Mol Liq 243:395–405
Saleem J, Shahid UB, Hijab M, Mackey H, McKay G (2019) Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers Biorefinery 9(4):775–802. https://doi.org/10.1007/s13399-019-00473-7
Sankararamakrishnan N, Gupta A, Vidyarthi SR (2014) Enhanced arsenic removal at neutral pH using functionalized multiwalled carbon nanotubes. J Environ Chem Eng 2:802–810
Schwinger SP, Fraga-García P, Eigenfeld M, Becker TM, Berensmeier S (2019) Magnetic separation in bioprocessing beyond the analytical scale: from biotechnology to the food industry. Front Bioeng Biotechnol 7:1–12
Shabnam R et al (2017) Novel magnetically doped epoxide functional cross-linked hydrophobic poly(lauryl methacrylate) composite polymer particles for removal of As(III) from aqueous solution. Ind Eng Chem Res 56:7747–7756
Shaji E, Santosh M, Sarath KV, Prakash P, Deepch V, Divya BV (2021) Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci Front 12(3):101079
Shankar S, Shanker U, Shikha (2014) Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci World J Article ID 304524. https://doi.org/10.1155/2014/304524
Sharma B, Thakur S, Trace D, Nezhad HY, Thakur VK (2020) Microwave-assisted rapid synthesis of reduced graphene oxide-based gum tragacanth hydrogel nanocomposite for heavy metal ions adsorption. Nanomaterials 10:1616. https://doi.org/10.3390/nano10081616
Sharma B, Thakur S, Mamba G, Prateek G, Gupta RK, Gupta VK, Thakur VK (2021) Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J Environ Chem Eng 9(1):10460
Sheila AIA, Raman AAA, Bello MM, Buthiyappan A (2019) Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J Environ Manage 246:547–556
Sheng G, Li Y, Yang X, Ren X, Yang S, Hu J, Wang X (2012) Efficient removal of arsenate by versatile magnetic graphene oxide composites. RSC Adv 2:12400–12407
Siddiqui SI, Chaudhry SA (2017) Iron oxide and its modified forms as an adsorbent for arsenic removal: a comprehensive recent advancement. Process Saf Environ 111:592–626
Sikder MT, Tanaka S, Saito T, Kurasaki M (2014) Application of zero-valent iron impregnated chitosan-carboxymethyl β-cyclodextrin composite beads as arsenic sorbent. J Environ Chem Eng 2:370–376
Sips R (1948) The Structure of a Catalyst Surface. J Phys Chem 16:490–495
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Srinivasan R (2011) Advances in application of natural clay and its composites in removal of biological, organic, and inorganic contaminants from drinking water. Adv Mater Sci Eng 2011:1–17. https://doi.org/10.1155/2011/872531
Tamaddoni Moghaddam S, Naimi-Jamal MR, Rohlwing A, Hussein FB, Abu-Zahra N (2019) High removal capacity of arsenic from drinking water using modified magnetic polyurethane foam nanocomposites. J Polym Environ 27(7):1497–1504. https://doi.org/10.1007/s10924-019-01446-7
Tang W, Li Q, Gao S, Shang JK (2011) Arsenic (III, V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J Hazard Mater 192(1):131–138
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch, A. (eds) Molecular, clinical and environmental toxicology. Experientia Supplementum 101:133–164. Springer, Basel. https://doi.org/10.1007/978-3-7643-8340-4_6
Tresintsi S, Mitrakas M, Simeonidis K, Kostoglou M (2015) Kinetic modeling of AS (III) and AS (V) adsorption by a novel tetravalent manganese feroxyhyte. J Colloid Interface Sci 460:1–7
Thakur S, Chaudhary J, SinghWalaa P, AlsanieSotirios F, Grammatikos A, Thakur VK (2022) Synthesis of bio-based monomers and polymers using microbes for a sustainable bioeconomy. Bioresource Technol 344(Part A):126156.
Thostenson ET, Ren Z, Chou T-W (2001) Advances in the science and technology of carbon nanotubes and their composites: a review. Compos Sci Technol 61(13):1899–1912. https://doi.org/10.1016/s0266-3538(01)00094-x
Thy LTM, Thuong NH, Tu TH, My NHT, Tuong HHP, Nam HM, Phong MT, Hieu NH (2020) Fabrication and adsorption properties of magnetic graphene oxide nanocomposites for removal of arsenic (V) from water. Adsorpt Sci Technol 38(7–8):240–253
Toth J (1971) State equation of the solid-gas interface layers. Acta Chimica Academiae Scientarium Hungaricae 69:311–317
Tripathy M, Adhikari S, Hota G (2020) L-Cysteine-functionalized mesoporous magnetite nanospheres: synthesis and adsorptive application toward arsenic remediation. J Chem Eng Data 65(8):3906–3919
Usman M, Katsoyiannis I, Mitrakas M, Zouboulis A, Ernst M (2018) Performance evaluation of small sized powdered ferric hydroxide as arsenic adsorbent. Water 10(7):957
Usman M, Zarebanadkouki M, Waseem M, Katsoyiannis IA, Ernst M (2020) Mathematical modeling of arsenic (V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J Hazard Mater 400:123221
Usman M, Katsoyiannis I, Rodrigues JH, Ernst M (2021) Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ Sci Pollut Res 28(42):59063–59075
Usman M, Belkasmi AI, Kastoyiannis IA, Ernst M (2021) Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: application study and mathematical modeling. J Chem Technol Biotechnol 96(6):1504–1514
Vu HC, Dwivedi AD, Le TT, Seo SH, Kim EJ, Chang YS (2017) Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: role of crosslinking metal cations in pH control. Chem Eng J 307:220–229
Wadhawan S, Jain A, Nayyar J, Mehta SK (2020) Role of nanomaterials as adsorbents in heavy metal ion removal from wastewater: a review. J Water Process Eng 33:101038
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As(III) and As(V) from aqueous solutions using nanoscale zero-valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Wang S, Gao B, Li Y, Creamer AE, He F (2017) Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: batch and continuous flow tests. J Hazard Mater 322:172–181
Wang J, Zhang W, Zheng Y, Zhang N, Zhang C (2019) Multi-functionalization of magnetic graphene by surface-initiated ICAR ATRP mediated by polydopamine chemistry for Adsorption and speciation of arsenic. Appl Surf Sci 478:15–25
Wang Y, Gao Y, Zhu Z, Zhang L, Zhao N, Fang Y, Zhu Y, Liu G (2021) Enhanced arsenic removal from aqueous solution by Fe/MN-C layered double hydroxide composite. Adsorption Sc Tech 2021:1–12
Wen Z, Dai C, Zhu Y, Zhang Y (2015) Synthesis of ordered mesoporous iron-manganese bimetal oxides for arsenic removal from aqueous solutions. RSC Adv 5:4058–4068
World Health Organization (1984) Guidelines for drinking water quality, recommendations, vol 1. WHO, Geneva, Switzerland, p 79
Yadav VB, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manage 232:803–817. https://doi.org/10.1016/j.jenvman.2018.11.120
Yang JC, Yin XB (2017) CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci Rep 7:40955
Yang MH, Zang YS, Huang H, Chen K, Li B, Sun GY, Zhao XW (2014) Arsenic trioxide exerts anti-lung cancer activity by inhibiting angiogenesis. Curr Cancer Drug Targets 14(6):557–566
Yu X, Tong S, Ge M, Zuo J, Cao C, Song W (2013) One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J Mater Chem A 1:959–965
Yürüm A, Kocabas-Atakli ZO, Sezen M, Semiat R, Yürüm Y (2014) Fast deposition of porous iron oxide on activated carbon by microwave heating and arsenic (V) removal from water. Chem Eng J 242:321–332
Zeng H, Zhai L, Qiao T et al (2020) Efficient removal of As(V) from aqueous media by magnetic nanoparticles prepared with iron-containing water treatment residuals. Sci Rep 10:9335. https://doi.org/10.1038/s41598-020-65840-1
Zeng H, Zhai L, Zhang J, Li D (2021) As(V) adsorption by a novel core-shell magnetic nanoparticles prepared with iron-containing water treatment residuals. Sci Total Environ 753:142002
Zhang J, Robert S (2005) Slow adsorption reaction between arsenic species and goethite (-FeOOH): diffusion or heterogeneous surface reaction control. Langmuir 21:2895–2901
Zhang S, Niu H, Cai Y, Zhao X, Shi Y (2010) Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem Eng J 158:599–607
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9(26):3562–3582. https://doi.org/10.1039/c8py00484f
Zhong LS, Hu JS, Liang HP, Cao AM, Song WG, Wan LJ (2006) Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv Mater 18(18):2426–2431
Zhu H, Jia Y, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172:1591–1596
Acknowledgements
The authors are thankful to the Director, National Institute of Technology Silchar, for his help and continuous support for the preparation of the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Mohammed Ahmaruzzaman alone has contributed in conducting the literature review and compilation of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmaruzzaman, M. Magnetic nanocomposite adsorbents for abatement of arsenic species from water and wastewater. Environ Sci Pollut Res 29, 82681–82708 (2022). https://doi.org/10.1007/s11356-022-23357-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23357-2