Abstract
Owing to the increase of pollutant sources in oceans, seas, and lakes, there is an expected effect on growth and metabolism of planktonic algae which are considered primary producers in the ecosystem. Therefore, it becomes urgent to carry out laboratory studies to test to what extent these pollutants can affect the growth of algae which is necessary as a food for marine fishes. Spirulina is considered the most important algal species due to its high nutritional value for humans and animals. Therefore, this work investigated the effect of different concentrations of Ni2+, Zn2+, and Cu2+ metal ion pollutants on growth of the blue-green alga Spirulina platensis. EC50 was identified to be around 2 mg/l for the three heavy metals. The suitability of Idku Lake for Spirulina platensis growth was investigated using multi-criteria spatial modeling integrated with remotely sensed data processing. Spatial distribution maps of turbidity, water nutrients, and phytoplankton were the input criteria used to assess Idku Lake’s suitability. The results obtained proved that low concentrations of the tested heavy metals stimulated growth and pigment fractions (chlorophyll a, carotenoids, and total phycobilins content) but to different degrees. The inhibitory effect was more prominent in the case of copper ions than zinc and nickel ions with all concentrations used. The overall suitability map of Spirulina platensis in Idku Lake showed that the whole lake is suitable for growth and proliferation except for the northwestern corner due to the high salinity levels. The present paper helps to understand the behavior of algae responding to environmental pollution, which supports environmental planners with the necessary baseline for investigating the fate of pollutants and the potential risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems perform numerous important environmental functions. For instance, they purify water, attenuate floods, recycle nutrients, recharge groundwater, and also afford habitats for wildlife. The aquatic environment is one of the significant media that should be protected from pollution. Approximately 1500 substances have been identified as pollutants in aquatic ecosystems, for instance, acids and alkalis, anions (e.g. sulfide, sulfite cyanide) detergents, municipal sewage and farm manures, wastes of food processing, gases (e.g. chlorine, ammonia), heat, heavy metals (e.g., lead, cadmium), nutrients particularly phosphates and nitrates, oil and oil dispersants, organic toxic wastes (e.g., formaldehydes, phenols), pathogens, pesticides, polychlorinated biphenyls, and radionuclide. Whether or not a compound will exert an effect on an organism or a community will depend on the concentration of that compound and the time of exposure to the compound. The effect of a pollutant on a target organism may be either acute or chronic (Saunder 1976).
The essential heavy metals (e.g., Cu, Fe, Mn, Se, Zn, etc.) are important for biochemical and physiological functions in living organisms (Tchounwou et al. 2012). Copper, for instance, is one of the valuable metals in many industrial processes, e.g., textile, painting, metal finishing, electroplating, and plastics (Al-Saydeh et al. 2017). In addition to copper, its effluents contain other elements, in lower concentrations, such as iron, zinc, nickel, etc. These metals may also have an undesirable impact on living organisms and the environment. Accordingly, copper-contaminated effluents have to be treated before discharge into natural water bodies. The common methods developed by microorganisms for removing from the environment include biotransformation, bioaccumulation, biomineralization, and biosorption (Ayangbenro and Babalola 2017; Cepoi et al. 2020).
Algal communities have been utilized in tests of toxicity for environmental monitoring of heavy metal pollution and can be used in determining general water quality and growth-limiting nutrients. Likewise, they can be used in the removal of metals from contaminated wastewater (Terry and Stone 2002). Marine phytoplankton is vital for the normal functioning of ecosystems since it forms the basis of the marine food chain. The disturbance to this component is likely to exert influences on higher trophic levels, which may be due to the release and accumulation of toxic compounds (Arora et al. 2002).
The genus Spirulina (Arthrospira) is considered one of the microalgae having commercial importance. Its biomass is extensively applicable in biofertilizers, feed, cosmetics, biofuels, food, and biomaterials. Spirulina is distinguished from other foods, since it grows in extreme environments. It grows in very alkaline water (> pH 9) where no other plant can grow, i.e., it does not require pesticides or herbicides. This means that Spirulina forming in the long term is the best and softest method to produce healthy foods without destroying the environment (Costa et al. 2019). Spirulina is a healthy whole food alga where it can supply the human body with the majority of essential elements and vitamins. The covering cell wall is not cellulose but a thin membrane that is composed of complex sugars which dissolve directly in the digestive juice of the stomach. Spirulina is considered a good food to sustain health and it is a good aid to regain health. Its metabolic products are quite sufficient to cover all these two items. It gets its own sun protective defense from the higher β-carotene production in the intensive sun. The hotter and stronger the sun shines, the better the quality of the product. Therefore, for Spirulina, nature itself is the best physician; it has a high nutritional value associated with its vitamin, mineral, and fatty acid content which are important for different biological activities, and easy digestibility (Lupatini et al. 2017; Trivedi et al. 2015). Spirulina contains several minerals such as magnesium, calcium, iron, and phosphorus. It represents a main source of iron as it contains 20 times more iron than a wheat gram (Cuellar-Bermudez et al. 2015; Ravindran et al. 2016).
Photosynthetic pigments in Spirulina are very important in different fields. Chlorophyll is one of the important pigments in Spirulina; it plays an important role in light capturing and contributes to anti-oxidants activities in algae, also it is used as a colorant in food and different pharmaceutical industries (Yuliani et al. 2019). Carotenoids have a valuable effect to influence the signaling and regulation of many biological pathways. Moreover, they have significant antioxidant activities (Ranga et al. 2018). In plants, carotenoids are excellent scavengers of singlet oxygen; thus, they protect cellular components, such as chlorophylls, lipids, proteins, and DNA, from oxidative damage in cells. Carotenoids are very important to human health; they enhance the immune system function and play important roles in the protection of different diseases (Raposo et al. 2015; Wang et al. 2002). Carotenoids, chlorophyll derivatives, and phycobilins are among the antioxidants with the highest activity in Spirulina sp. (Jaime et al. 2005).
Adding small amounts of microalgae biomass is valuable for the physiology and the immune response of animals. It also boosts antiviral resistance, antibacterial, and intestinal function. These factors result in growth promotion, weight control, improved feed conversion, and reproductive performance (Harel et al. 2007).
Spirulina platensis is an un-branched filamentous blue-green alga attaining the size of 0.5 mm in length and occurs naturally in highly alkaline lakes. Spirulina sp. is present in different Egyptian lakes especially Lake Mariut and Lake Idku. Lake Mariut is extensively polluted as it suffered from serious ecological damage due to industrialization and modern agricultural systems (Abd El-Hamid et al. 2021). As a result of the extensive nutrients input received from Alexandria city, the enclosed nature of the lake and the water shallowness, heavy algal blooms, and domination of plankton occurred particularly Spirulina sp. On the other hand, Lake Idku is characterized by high nutrient concentrations of NO2-N (5.4 μg/l), NH4-N (4.5 μg/l), PO4-P (7.8 μg/l), and SiO2-Si (97.7 μg/l) approximately, as well as a high level of dissolved oxygen (8.3 mg/l). This is mostly attributed to the excessive amounts wastewater discharged into the lake (Khairy et al. 2015). This alga used in this research was kindly provided by Prof. Yahia Azab, Algal Culture Collection of El-Mansoura University, Egypt. Spirulina platensis was grown in Spirulina medium (Zarrouk 1966).
Applications of remote sensing and geographic information system are widely used in environmental assessment studies. Extracting environmental variables from remotely sensed imagery is important to facilitate studying regional and inaccessible areas which cannot be investigated using traditional methods. The integration of remote sensing with spatial modeling helps for environmental site selection studies (Effat and El-Zeiny 2017; 2020; El-Zeiny and Effat 2019; Al-Shaibah et al. 2021).
Recently, several research studies in Egypt have been carried out to investigate different environmental components associated with biological factors such as Dunaliella algae, mosquito, and wild plant habitats (El Agawany et al. 2021; El-Amier et al. 2021; Nagy et al. 2021).
The spatial distribution maps using geostatistical analyses represent one of the most common applications of geographic information systems. These analyses help to generate continuous surfaces by predicting values based on real measurements (El-Zeiny et al. 2019). Generation of geographic databases with all available resources and potential hazards is important for decision-making process Therefore, present study aims to assess the effects of different heavy metals concentrations on the growth and photosynthetic pigmentation of Spirulina platensis. Furthermore, the suitability of Idku Lake for the proliferation of Spirulina platensis is tested seeking for identifying the most suitable part of the lake using the cartographic modeling techniques.
The new knowledge added from this paper can be summarized as scientific and economic-environmental knowledge. The scientific sector is the integration between lab analyses and spatial techniques to test the algae response to different levels of heavy metals and the suitability of Idku Lake for proliferation which represents a practical application to the lab experiment in a real case study. The economic-environmental knowledge can be expressed in resolving a severe environmental problem through Spirulina algae indicating biological treatment with the proliferation of the algae with an economic value.
Materials and methods
I-Biological material
In the present study, the tested biological material was the axenic filamentous cyanobacterial alga Spirulina platensis. This alga was kindly provided by Prof. Yahia Azab, Algal Culture Collection of El-Mansoura University, Egypt. Spirulina platensis was grown in Spirulina medium (Zarrouk 1966) with specific component as shown in Table 1.
I-i-Growth measurements by optical density
The growth of the investigated alga (Spirulina platensis) was determined by optical density which was determined at 488 nm and compared to calibration standard curve. The optical density was calculated according to the following equation (Robert 1979):
where: I is the transmittance of sample. IO is the transmittance of blank adjusted to read 100%.
However, the following equations were also used for calculation of the mean growth rate, growth rate, and logistic growth model from the obtained O.D (biomass).
I-ii-Growth rate
The growth rate is calculated from the equation:
where: B1 is the optical density at time t1. B0 is the optical density at time t2. t1 is the time at the beginning of the experiment. t2 is the time at the end of the experiment.
Mean growth rate
The mean growth rate is calculated from the equation:
where: Bt is the optical density at time t. B0 is the optical density at the beginning of the experiment.
I-iii-Logistic growth model
The maximum growth rate (r max) is calculated at the exponential phase of growth from the equation of growth rate. After that, r max was used to calculate the whole sigmoid growth where growth rate (r) at any period of time:
where: B is the optical density. K is the carrying capacity (maximum yield).
Then r max, K, and X0 (initial biomass) were used to calculate change in biomass using sigmoidal growth model.
II- Estimation of photosynthetic pigments
i- Chlorophyll “a”
The spectrophotometric method is the easier method for measuring chlorophyll “a” and the results calculated according to the trichromatic equation of Jeffrey and Humphrey (1975):
where: Va is the volume of the extract. Vs is the volume of the algal suspension.
ii- Carotenoids
The spectrophotometer method suggested by Jensen and Liaaen (1959) was used in this investigation. The concentration of carotenoids was calculated using the following equation:
where: C is the concentration of carotenoids (mg.l−1). D is the absorbance at 450 nm. F is the dilution factor. V is the volume of algal suspension. 2500 is an average of extinction coefficient.
iii- Phycobilins
Phycobilins were determined according to the method described by Bennett and Bogorad (1973). The concentrations of phycocyanin, allophycocyanin, and phycoerythrin in crude extracts were calculated as mg/l by using the following equations:
III-Preparation of different concentrations of nickel, copper, and zinc
Three heavy metals, namely nickel, copper, and zinc, were selected during the present study based on their abundance in wastewater as well as their effect on the receiving aquatic ecosystems. Stock solutions of the selected heavy metals were prepared from their salts in double distilled water and sterilized by filtration through 0.2 µm nitrocellulose membranes. The different concentrations of the used selective heavy metals in the metal bioassays were prepared by dilution with double distilled water. As stated by Wong and Pak (1992), an initial experiment using a varied range of metal solutions (NiCl2, CuSO4.5H2O, and ZnCl2) was achieved to identify the appropriate concentrations of these metal salts that could be tolerated by the investigated alga. Selections of a series of concentrations were based on the response of the studied alga which had a slightly or noticeable impacts on its growth, and also to exclude the non-effective and directly deadly concentrations on the experimented alga. Consequently, the following concentrations were selected: 1.0, 1.5, 2.0, 2.5, and 3.0 mg/l respectively for each heavy metal.
IV-Suitability of Idku Lake for Spirulina platensis and satellite imagery analyses
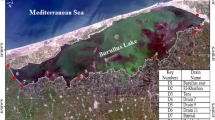
The suitability of Idku Lake was evaluated for proliferation of Spirulina platensis using geospatial techniques. Idku Lake was chosen as one of the highly polluted Egyptian lakes which receives saline water from the Mediterranean Sea through different water inlets, namely “Boughaz” and receives freshwater from different fresh drains distributed along with the southern parts of the Lake (Fig. 1).
On basis of the optimal water quality parameters of Spirulina platensis, the suitability maps were produced. Suitability is increased with the elevation in alkalinity, phosphate, nitrate, nitrite, phosphate, ammonia, and with the decline of TDS and turbidity. A recent multispectral Landsat image dated 17 March 2021 was preprocessed to calibrate the data to produce the NDVI which was used for the identification of phytoplankton content of the lake; higher levels of NDVI indicate more concentration of chlorophyll. Radiometric correction, dark object subtraction, and spatial subset were the preprocessing steps followed to calibrate the data and convert it into reflectance for further processing including indices calculation. NDVI was calculated from the red and NIR bands: NDVI = (NIR − Red)/(NIR + Red). Thus, suitability map for each single parameter was generated and converted into a “raster” layer. These layers were reclassified and inserted into a weighted overlay model to identify the most suitable part of the lake considering all investigated variables (Fig. 2).
Results and discussions
In our country, we have little information about the ambient concentrations of heavy metals. It is not correct to rely on the data present owing to the presence of many different polluted regions. Heavy metals contamination in water has a seriously harmful effect on living organisms (i.e., Flora and Fauna). Thus, the system of the present work was partitioned into two parts:
Part I
In this part, preliminary experiments were conducted on the effect of the three selected heavy metals Ni2+, Zn2+, and Cu2+ on the growth of Spirulina platensis in order to choose the suitable concentrations that could be safely tested (Figs. 3, 4, and 5). The organism was firstly cultured under selected concentrations of these heavy metals, namely 25.0, 22.5, 20.0, 17.5, and 15.0 mg/l. The obtained results cleared that the organism differently responded under the stress effect of these heavy metal ions and died after 4 days of culturing. The determinable effects were clearer in case of Cu than in the other two heavy metal ions. Therefore, the foregoing experiments were carried out under fewer concentrations than in the case of the first experiment. These concentrations were 12.5, 10.0, 7.5, 6.5, and 5.0 mg/l.
These results go with those obtained by Nalimova et al. (2005) who found that the lethal effect of Cu and Zn on Spirulina platensis was 5 and 8.8 mg/l respectively. The results obtained cleared that a sudden drop in growth of the tested organism was recorded at the 8th day of culturing. The organism appeared pale in color and dropped in the bottom of the culturing flasks especially at Cu and Zn elements. Finally, a third experiment was carried out at concentrations of 3.0, 2.5, 2.0, 1.5, and 1.0 mg/l for Ni, Zn, and Cu ions. Under these selected concentrations, S. platensis remained alive but with different rates of growth. The results cleared also that the effective concentration (EC50) of Ni, Zn, and Cu was recorded nearly at a concentration 2.0 mg/l after 8 days of culturing. Similar results obtained by Nalimova et al. (2005) who found that the addition of 2.55 mg/l Cu2+ on the second day of culturing increased culture growth and when Cu2+ levels were increased to 3.8 mg/l, the growth rate and culture productivity decreased on the fourth day. Consequently in this work, five concentrations were chosen (two concentrations below and two higher than 2.0 mg/l), i.e., l.0, 1.5, 2.0, 2.5, and 3.0 mg/l for each element beside control.
Part II
This part was concerned with the effect of the five different concentrations of Ni, Zn, and Cu that have been chosen from part I on growth and content of some related metabolic compounds of S. platensis cells.
Effect of different concentrations of Ni2+, Zn2+, and Cu.2+ ions on growth of Spirulina platensis measured as optical density
Risk assessment is a continually evolving process as important information on different contaminants especially heavy metals, the health effects involved, and their occurrence in food are all factors that should be continuously measured and monitored. In correlation with the results obtained by using optical density (O.D.), parameters of growth of Spirulina platensis at the control and under the effect of the different concentrations of Ni, Zn, and Cu were recorded in Figs. 6, 7, and 8. With regard to the growth parameters based on O.D. (growth rate and mean growth rate) cleared that maximum growth rate and maximum mean growth rate were obtained on the 8th day of culturing for all the concentrations of these three tested elements but with different values, these results coincide with those obtained by El-Maghrabi (1997). For Ni, the maximum growth rate at the 8th day of culturing reached 0.498, 0.466, 0.415, 0.385, and 0.325 mg/l at concentrations 1.0, 1.5, 2.0, 2.5, and 3.0 mg/l, respectively. For Zn and under the same tested concentrations, the maximum rate of growth reached 0.497, 0.465, 0.421, 0.376, and 0. 312 mg/l while for Cu, these values reached 0.493, 0.461, 0.416, 0.335, and 0.294 mg/l, respectively. Spirulina alga has a high ability to bind metal ions from solution and bind with heavy metals due to the presence of functional groups that can bind with metal ions. The functional groups, especially carboxyl groups, amine, hydroxyl, and sulfate, are contained in the cytoplasm of the cell wall (Budi et al. 2020).
As pointed out previously, it appeared that the rate of growth was lessened in case of Zn and Cu than in case of Ni. The same trend was also recorded at the other concentrations of the three tested elements. This means that within the same element, the rate of growth was affected by the concentration of the element tested and the length of the culture period. Moreover, the results obtained proved that Cu ions whether at low or high concentrations were more toxic than both Ni and Zn ions.
Our results also go with the harmony of those explained by Soni et al. (2017) who observed that under stressed conditions, there was a significant change in the functional properties of Spirulina. Environmental stresses like high pH, light, salinity, temperature, nitrogen concentration, and different types of pollutants affect growth and nutrient productivity.
Ni2+ ions are known to be essential cofactors for four bacterial enzymes (Thauer 1983). Our results revealed that at lower concentrations of Ni (1.0 mg/l), the growth of S. platensis stimulated or enhanced, while the growth decreased and reached the lower values concentration at the maximum concentration of Ni (3.0 mg/l). Similar results were also obtained by Stratton and Corke (1979) who reported that high concentrations of Ni2+ were also toxic to Chlorella and Anabaena species. Furthermore, Stillwell and Holland (1977) proved that the rate of cell division in Cricosphaera carterae decreased progressively by increased nickel concentration.
The obtained results revealed also that S. platensis was able to display remarkable improvement at the worked lower concentration of nickel. In addition, Rai and Raizada (1986) observed maximum stimulation of growth in Nostoc muscorum at low concentrations of nickel. Besides, El-Mazally (2002) found that low concentrations of Ni2+ stimulated the growth of Scenedesmus obliques compared to control. Therefore, lower nickel values played a noteworthy role in the variation of biomass. The obtained results go in harmony with those obtained by Van Baalen and O’Donnell (1978) who found that the growth of one species of Cyanophyta has been reported to be dependent on nickel. Accumulation of nickel ions can be related to their intracellular gathering and fixation by spirulina biomass to decrease the toxicity (Zinicovscaia et al. 2016).
The results concerning the effect of different concentrations of Zn2+ ions on the growth of S. platensis cleared that Zn2+ ions are more toxic than Ni2+ ions on the growth of the alga. At lower concentrations (1.0 mg/l Zn2+), the rate of growth increased by 6.036% compared to control. The same result was observed by Cepoi et al. (2020) who indicated that S. platensis accumulates Zn in the biomass in amounts that exceeds the optimal amount determined for the growth. Zinc has great importance since it plays an important role in gene regulation and is involved in protein, nucleic acid, lipid, and carbohydrate metabolism. It is also considered an essential microelement for almost all classes of organisms (Ishimaru et al. 2011).
On the contrary, at concentrations 2.0, 2.5, and 3.0 mg/l Zn2+, the rate of growth decreased from the 10th day of culturing to the end of the experiment but the rate of decrease was more prominent at 3.0 mg/l than at 2.0 and 2.5 mg/l Zn2+. These results nearly coincide with those obtained by many authors: Fisher et al. (1981) reported an increase in growth of Asteriorella japonica responding to high copper and zinc levels, El-Naggar (1993) stated that high levels of zinc cause reduction in the growth of Chlorella vulgaris and Scenedesmus bijuga. Nalimova et al. (2005) reported that the increase in zinc concentrations (4.4 and 6.6 mg/l) reduced culture productivity of Spirulia platensis because zinc affects practically all physiological processes (i.e., membrane functioning, photosynthesis, cell division, and respiration). Similarly, Zinicovscaia et al. (2021) observed that S. platensis accumulated 2.5 mg Zn/g at zinc concentration in solution 2.5 mg/l.
The work presented here provides also that all tested concentrations of Cu2+ inhibited the growth of S. platensis measured as optical density but with different degrees. The lowest concentration (1.0 mg/l) accelerated the growth but increasing concentration of copper inhibited algal growth. Our results go in agreement with those obtained by Budi et al. (2020) who indicated that with the treatment of various levels of Cu, the extreme growth rate of Spirulina plantesis displays that the treatment by adding 1 ppm heavy metal is needed for increasing growth but the higher the concentration of Cu given, the lower the density of Spirulina plantesis. The importance of Cu2+ as an essential micro-nutrient and its effect in limiting algal growth and related metabolic activities was reported by many authors (Rai et al. 1991; Vymazal 1995; Budi et al. 2020).
Although Cu2+ is significant metal for living organisms, it can be lethal and cause the death of algal cells at high levels, and a decrease in growth and some important metabolites depended on increasing Cu concentration (Mohy El-Din 2017). Correspondingly, Stauber and Florence (1985) stated that this metal at levels greater than 5 mg/l reduced the growth of N. closterium by 50% below control. Copper was found more poisonous to the dinoflagellate Prorocentrum micans than the diatom (Nitzschia closterium) as reported by Carpene and Boni (1992). Furthermore, the poisonous copper effect on the growth of marine alga Dunaliella tertiolecta was obviously demonstrated in the cultures treated with the copper of 10 and 12 mg/l as recorded by Abalad et al. (1995).
Inhibition of algal growth by copper was reported in Anabaena doliolum (Rai et al. 1991 b), Nostoc calcicola, Nostoc muscorum (Pandy and Chatterjee 1999), Chlorella vulgaris (Brock 1969), and diatoms (Pistocchi et al. 2000). Furthermore, the results display that the inhibitory copper effect on the growth rate is higher pronounced than the other experienced metals (nickel and zinc). These findings are matching with several previously published results (Muwafq and Bernd 2006). Meenakshi (2007) observed high reduction in growth of Spirulina paltensis culture, and copper toxicity was higher than zinc by using 2 mg/l for both heavy metals. The toxicity of heavy metals may be remarked by changes in growth conditions and reduction in the growth of the tested microorganism.
The logistic growth model proposed in this study can be used effectively to describe the results of algae inhibition growth tests (Figs. 9, 10, and 11). They do not have more factors than the routine analysis related to the logistic growth model which relates the population intrinsic growth rate to the heavy metal concentration. Wang et al. (2002) reported that the logistic growth model is better for in situ algal growth owing to the slow growth in the early phase and limited resources.
Effect of different concentrations of Ni2+, Zn2,+ and Cu.2+ ions on photosynthetic pigment fractions of Spirulina platensis
Data concerning the effect of different concentrations of Ni, Zn, and Cu on content of chlorophyll a, carotenoids, and total phycobilins in S. platensis cultured for 18 days were represented in Figs. 12, 13, and 14. Anent data obtained for quantitative and for chlorophyll content as another parameter for growth determination concluded that the obtained results support also those obtained for optical density, i.e., maximum values of growth were recorded on the 10th day. This may support the idea that chlorophylls especially chlorophyll “a” could be used as a good parameter for growth determination. It must be mentioned here that chlorophyll content increased gradually with increasing the period of culturing till nearly the 10th day of the experiment. In this case, the results could be used only for constructing the growth curve of the organism. This means that in spite of the fact that optical density increased gradually with increasing the period of culturing, the content of chlorophyll decreased. This coincides with the results obtained by Markovits et al. (1993) who indicated that amount of chlorophyll decreased with the increase in culturing period together with the decrease of the available nutrients in the medium.
The present study indicates that application of low Ni2+ concentrations to cultures of S. platensis causes a significant increase in different pigment fractions (chlorophyll “a” phycobilins and carotenoids). On the other hand, higher Ni2+ concentrations suppressed the levels of these pigment fractions. These findings are in agreement with data obtained by Angadi and Mathad (1994) who found that chlorophylls were maximally inhibited at higher concentrations of Ni2+, but were stimulated at lower concentrations. Furthermore, similar results were obtained by El-Mazally (2002) for Monoraphidium minutum and Scenedesmus obliques.
These data proved that values of these parameters are differed according to concentrations of Zn and cleared also that Zn is more toxic than Ni ions. By increasing period of culturing, the toxicity of Zn was more prominent at higher concentrations 2.0, 2.5, and 3.0 mg/l than lower ones (1.0 and 1.5 mg/l). It is clear also that at higher concentrations of Zn, the decrease in the content of chlorophyll a and carotenoids was more prominent than that of phycobilins.
Chlorophyll “a” content followed nearly a similar pattern of change to that of growth responding to diverse concentrations of zinc. El-Naggar (1993) indicated that lower concentrations of zinc stimulate growth and increase the different pigment fractions of the green alga Chlorella vulgaris and Scenedesmus bijuga. However, higher concentrations of zinc suppressed the level of pigment fractions. Our results go with harmony with those obtained by Prassad and Prassad (1987) who found that low Zn2+ levels improved the total chlorophyll content in Asterionella japonica. High levels of heavy metals hinder the enzymes that are responsible for the chlorophyll synthesis. Zn2+ does not directly accelerate the reactive oxygen species formation owing to its redox inertness. Therefore, it exerted comparatively less stress on the investigated organism. Higher Zn2+ concentrations lead to decrease in cell division and total chlorophyll content (Afkar et al. 2010). The results cleared also that, the percentage of decrease in chlorophyll a content differed according to concentrations used and period of culturing. These results are in harmony with those obtained by Ghezelbash et al. (2008) who represented that reduction in chlorophyll content with reduced growth rate is due to decrease in photosynthetic rate.
Using different concentrations of Cu gave different values for the content of these pigment fractions (chlorophyll a, carotenoids, and total phycobilins). The results cleared also that Cu ions are more toxic to the content of pigment fractions of S. platensis than both Ni and Zn ions. In this study, the stimulatory effect of copper recorded during the first 2 days of culturing with lower concentrations can be accounted for either as a result of algal requirement of this element in metabolic processes or clarified by production of some organic compound which reduces metal toxicity. The poisonous effect of copper at higher concentrations may arise from the oxidative potential of copper (II) that causes reduction of chlorophyll (Muwafq and Bernd 2006). Our results are proven by those obtained by Budi et al. (2020) who recorded that 5 ppm Cu2+ is considered being a strong growth inhibitor of Spirulina plantesis due to inhibition of respiration for photosynthetic microalgae. Likewise, our results go matching with those obtained by Cepoi et al. (2020) who reported that copper toxicity for cyanobacteria can be explained by production of reactive oxygen species which leads to severe damage of lipids, proteins, and DNA and makes the substitution of Mg in chlorophyll. The inhibition in photosynthetic pigments accumulation responding to heavy metal stress may be also a magnitude of peroxidation of chloroplast membranes through the increased rate of reactive oxygen species production (Bajguz 2011).
Copper is an essential element for higher plants and algae (Breteler et al. 1984). Copper requirement could not be satisfied by any of other elements when ions of these elements were added singly to growth cultures lacking Cu2+ (Walker 1953). Copper is toxic to most organisms at high levels, therefore it is utilized in numerous molluscicides, fungicides, algicides, and marine antifouling compounds (Woolhouse 1983). Carotenoids and phycobilins content behaved the same way as in case of chlorophyll a. The total carotenoids at the lowest concentration of Zn2+ (1.0 mg/l) significantly increased gradually over control till the 8th day of culturing. At the other concentrations of Zn2+, the content of carotenoids and phycobilins decreased gradually till the end of the experiment. In the case of Cu2+, the decrease in the content of carotenoids and phycobilins was more prominent than in Zn2+. These results go in harmony with those obtained by El-Maghrabi (2002).
Assessing Idku Lake for growth of Spirulina platenses
Statistics of water quality parameters of Idku Lake are shown in Table 2. Most of the investigated parameters showed a noticeable fluctuation due to the impact of drainage water discharged into the lake from multiple sources. Neutral to weak alkaline water samples were reported since pH ranged from 7.30 to 8.60 with a mean of 7.86. High to low salinity water samples were observed due to the impact of saline seawater in the North and drainage freshwater in the South where TDS ranged from 1438 to 42,409 with a mean of 14,159 mg/l.
Nutrients showed high levels at the eastern parts of the lake which might be due to the appearance of some agricultural drains nearby these sites. A great fluctuation is observed in the levels of turbidity among different sites which ranged from 10.1 to 120 with a mean 30.01 NTU. The high levels of turbidity are dominating the lake due to suspended particles and contaminants received with wastewater of drains. NDVI was employed to assess phytoplankton of the lake which showed the highest density in majority of the lake showing mean value 0.27 indicating the sparse density inside the lake.
Based on the optimum conditions of the investigated parameters for the growth and spread of Spirulina platensis, the suitability spatial distribution maps were generated as shown in Fig. 15. Nitrate, nitrite, ammonia, salinity, and NDVI showed the same spatial patterns of suitability where eastern borders of the lake are more suitable than the western parts.
The overall suitability map of Spirulina platensis in Idku Lake showed that the whole lake is suitable for the growth and proliferation except the northwestern corner due to the high salinity levels (Fig. 16). NDVI showed high levels in majority of the lake which indicates the appearance of different algal species. This means that the environmental conditions of the lake are promising for the spread of various algal species such as Spirulina, considering the potential impact on aquatic fauna.
Conclusion
In Egypt, there is little information about the ambient concentrations of heavy metals. Algae are considered an excellent indicator for measuring different environmental pollutants. Risk assessment is a continually evolving process as important information on different contaminants, especially heavy metals. The health impacts involved and their occurrence in food are all factors that should be continuously measured and monitored. In this research, Spirulina platensis was selected for measuring the effect of different concentrations of three heavy metals (Ni, Zn, and Cu) on growth and photosynthetic pigment fractions due to the high importance of this type of algae. The suitability of Idku Lake was evaluated for proliferation of Spirulina platensis using geospatial techniques. Nitrate, nitrite, ammonia, salinity, and NDVI showed the same spatial patterns of suitability where the eastern borders of the lake are more suitable than the western parts. The results proved that the inhibitory effect of copper on the growth measured by optical density, growth rate, and mean growth rate was more pronounced than nickel and zinc under all the tested concentrations. In comparing toxicity of zinc and nickel, it was observed that Zn2+ ions are more toxic than Ni2+ ions. Lower concentrations of zinc and nickel stimulated growth and increased different pigment fractions (chlorophyll a, carotenoids, and total phycobilins content) while Cu2+ ions are more toxic to the content of pigment fractions than both Ni and Zn ions.
Data availability
All data generated or analyzed during this study are included and available in this article.
References
Abalad J, Cid A, Herrero C, Torres E (1995) Response of the marine micro-alga Dunaliella tertiolecta to copper toxicity in short time experiments. Bull Environ Contam Toxicology 54:317
Abd El-Hamid HT, El-Alfy MA, Elnaggar AA (2021) Prediction of future situation of land use/cover change and modeling sensitivity to pollution in Edku Lake, Egypt based on geospatial analyses. GeoJournal 86:1895–1913
Afkar E, Ababna H, Fathi AA (2010) Toxicological response of the green alga Chlorella vulgaris, to some heavy metals. Am J Environ Sci 6(3):230–237
Al-Saydeh SA, El-Naas MH, Zaid J (2017) Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem 56(25):35–44
Al-Shaibah B, Liu X, Zhang J, Tong Z, Zhang M, El-Zeiny A, Faichia C, Hussain M, Tayyab M (2021) Modeling water quality parameters using landsat multispectral images: a case study of Erlong Lake, Northeast China. Remote Sens 13:1603. https://doi.org/10.3390/rs13091603
Angadi SB, Mathad P (1994) Effect of chromium and nickel on Scenedesmus quadricauda (Turp.) de Berb Phykos. J Phycol Sci New Delhi 33(1 and 2):99–103
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial bio sorbents. Int J Environ Res Public Health 14(1):94
Bajguz A (2011) Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contam Toxicol 60:406–416
Bennett A, Bogorad L (1973) Complementary chromatic adaption in a filamentous blue-green algae. J Cell Biol 58(4):19–435
Breteler RJ, Rachlin JW, Engel DW (1984) Metals subpanel report. In: Breteler RJ (ed) Chemical pollution in the Hudson-Raritan Estuary, vol 7. NOAA Technical Memorandum NOS OMA, pp 12–35
Brock TD (1969) Microbial growth under extreme conditions. In: Meadow PM, Pirt SJ (eds) Microbial growth, 19th symposium soc. gen. Microbial. Cambridge University Press, London, pp 15–41
Budi RMS, Rahardja BS, Masithah ED (2020) Potential concentration of heavy metal copper (cu) and microalgae growth Spirulina plantesis in culture media. IOP Conf Ser Earth Environ Sci 441:012147
Carpene E, Boni L (1992) Effects of heavy metals on the algae Nitzschia closterium and Prorocentrum micans. Sci Total Environ (Netherlands) (126):921–927
Cepoi L, Zinicovscaiac I, Rudia L, Chiriaca T, Miscua V, Djura S, Strelkovac L, Vergelc K, Nekhoroshkovc P (2020) Growth and heavy metals accumulation by Spirulina platensis biomass from multicomponent copper containing synthetic effluents during repeated cultivation cycles. Ecol Eng 142:105637
Costa JA, Freitas BC, Rosa GM, Moraes L, Morais MG (2019) Operational and economic aspects of Spirulina-based biorefinery. Biores Technol 292:121946
Cuellar-Bermudez S, Garcia-Perez J, Rittmann B, Parra-Saldivar R (2015) Photosynthetic bioenergy utilizing CO2: an approach on flue gases utilization for third generation biofuels. J Clean Prod 98:53–65
Effat HA, El-Zeiny A (2017) Modeling potential zones for solar energy in Fayoum, Egypt, using satellite and spatial data. Model Earth Syst Environ 3(4):1529–1542. https://doi.org/10.1007/s40808-017-0372-2
Effat HA, El-Zeiny AM (2020) Integration of satellite data and spatial decision models for zoning new urban communities in El-Fayoum Desert. Arab J Geosci 13:1093. https://doi.org/10.1007/s12517-020-06031-0
El Agawany N, Kaamoush M, El-Zeiny A, Ahmed M (2021) Effect of heavy metals on protein content of marine unicellular green alga Dunaliella tertiolecta. Environ Monit Assess; 193 (584). https://doi.org/10.1007/s10661-021-09353-y
El-Amier YA, El-Zeiny A, El-Halawany E-SF, Elsayed A, El-Esawi MA, Noureldeen A, Darwish H, Al-Barty A, Elagami SA (2021) Environmental and stress analysis of wild plant habitat in River Nile Region of Dakahlia Governorate on basis of geospatial techniques. Sustainability 13:6377
El-Maghrabi DM (2002) Studies on the production of some important fatty acids from Algae. Ph.D. Thesis. Botany Department, Faculty of Science, Alexandria University, Egypt
El-Maghrabi DM (1997) The biotechnology of culturing Dunaliella salina for the production of some valuable metabolites. M.Sc. Thesis. Botany Department, Faculty of Science, Alexandria University, Egypt
El-Mazally EE (2002) Ecophysiological studies on the phytoplankton communities of River Nile at Kafr El-Zayat city with relevance to pollution with industrial effluents. Ph.D. Thesis. Botany Department, Faculty of Science, Tanta University, Egypt
El-Naggar AH (1993) Growth and some metabolic activities of Chlorella and Scenedesmus in relation to heavy metal pollution in Gharbia Governorate. Ph.D. Thesis. Botany Department, Faculty of Science, Tanta University, Egypt
El-Zeiny AM, Effat HA (2019) Environmental analysis of soil characteristics in El-Fayoum Governorate using geomatics approach. Environ Monit Assess 191:463. https://doi.org/10.1007/s10661-019-7587-9
El-Zeiny AM, El Kafrawy SB, Ahmed MH (2019) Geomatics based approach for assessing Qaroun Lake pollution. Egypt J Remote Sens Space Sci 22(3):279–296. https://doi.org/10.1016/j.ejrs.2019.07.003
Fisher NS, Jones GJ, Nelson DM (1981) Effects of copper and zinc on growth, morphology, and metabolism of Asterionella japonica (Clev.). J Exp Mar Biol Ecol 51:37–56
Ghezelbash F, Farboodnia T, Heidari R, Agh N (2008) Biochemical effects of different salinities and luminance on green microalgae Tetraselmis chuii. Research J Biology Sci 3(2):217–221
Harel M, Clayton D, Bullis RA (2007) Patent application publication – feed formulation. How to make sense? Renew Sustain Energy Rev 47:295–307
Ishimaru Y, Bashir K, Nishizawa NK (2011) Zn uptake and translocation in rice plants. Rice 4:21–27
Jaime L, Mendiola JA, Herrero M, Soler-Rivas C, Santoyo S, Señorans FJ, Cifuentes A, Ibáñez E (2005) Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J Separ Sci 28(16):2111–2119
Jeffrey SW, Humphrey GF (1975) New spectroscopic equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167:191–194
Jensen A, Liaaen S (1959) Quantitative paper chromatography of carotenoids. Acta Chem Scand 13:1813
Khairy HM, Shaltout KH, El-Sheekh MM, Eassa DI (2015) Algal diversity of the Mediterranean lakes in Egypt. In: International conference on Advances in Agricultural, Biological & Environmental Sciences (AABES-2015). London (UK)
Lupatini AL, Colla LM, Canan C, Colla E (2017) Potential application of microalga Spirulina platensis as a protein source. J Sci Food Agric 97:724–732
Markovits A, Gianelli MP, Cone JR, Eraso S (1993) Strain selection for β- carotene production by Dunaliella. Wold J Microbiol Biotechnol 9:534–537
Meenakshi B (2007) Bioremediation of oils: Role of Cyanobacteria. In: Biotechnological Applications of Microalgae. Narosa Publication House New Delhi, pp 211–243
Mohy El-Din SM (2017) Effect of copper and lead on growth and some metabolic activities of cyanobacterium Spirulina platensis (Nordstedt). Egypt J Bot 57(3):445–456
Muwafq HM, Bernd M (2006) Toxicity of heavy metals on Scenedesmus quadricauda (Turp.) de brebisson in batch cultures. Environ Sci Pollut Res 13:98–104
Wanga Na, Manabeb Y, Sugawarab T, Paulc NA, Zhaoa J (2018) Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem 242:247–255
Nagy A, El-Zeiny A, Elshaier M, Sowilem M, Atwa W (2021) Water quality assessment of mosquito breeding water localities in the Nile Valley of Giza Governorate. J Environ Sci 50(1):1–10
Nalimova AA, Popova VV, Tsoglin LN, Pronina NA (2005) The effects of copper and zinc on Spirulina platensis growth and heavy metal accumulation in its cells. Russ J Plant Physiol 52(2):229–234
Pandy U, Chatterjee C (1999) Response of two strains of Nostoc muscorum to metal stress and salinity. Annals of Applied Biology 134:259–263
Pistocchi R, Mormile AM, Guerrini F, Isani G, Boni L (2000) Increased production of extra- and intracellular metal-ligands in phytoplankton exposed to copper and cadmium. J Appl Phycol 12:469–477
Prassad D, Prassad A (1987) Alteredaminolaevulinic acid metabolism bylead and mercuryin germinating seedlings of Bajra (Pennisetum typhoideum). J Plant Physiol 127:241–249
Rai LC, Raizada M (1986) Nickel induced stimulation of growth, heterocyst differentiation, C-14 uptake and nitrogenase activity in Nostoc muscorum. J Indian Bot Soc 67:74–77
Rai LC, Singh JB, Mallick N, Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J of Plant Physiology 138(1):68–74
Ranga RA, Deepika G, Ravishankar GA, Sarada R, Panduranga NB, Lei B (2018) Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Crit Rev Food Sci Nutr 59(12):1880–1902
Raposo M, De Morais A, De Morais R (2015) Carotenoids from marine microalgae: a valuable natural source for the prevention of chronic diseases. Mar Drugs 13:5128–5155
Ravindran B, Gupta SK, Cho WM, Kim JK, Lee SR, Jeong KH, Lee DJ, HC (2016) Microalgae potential and multiple roles-current progress and future prospects - an overview. Sustainability 8(12)
Robert RLG (1979) Growth measurements. Division rate. In: Stein RJ (ed) Handbook of Physiological methods, culture methods and growth measurements. Cambridge University Press, London
Saunder PJW (1976) 1976. Manchester University Press, The estimation of pollution damage. Manchester University Manchester
Soni RA, Sudhakar K, Rana RS (2017) Spirulina from growth to nutritional product: a review. Trends Food Sci Technol 69(2017):157–171
Stauber JL, Florence TM (1985) Interaction of copper and manganese: a mechanism by which manganese alleviate copper toxicity to the marine diatoms (Her.) W. Smith. Aquatic Toxicol 7:241
Stillwell EF, Holland JR (1977) Nickel effects on cell division, calcification, and cell protein in the coccolithophorid Cricosphaera carterae Sci. Biol J 3:401
Stratton GW, Corke CT (1979) The effect of mercury, cadmium and nickel ion combinations on a blue green alga. Chemosphere 10:731–740
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Terry PA, Stone W (2002) Biosorption of cadmium and copper contaminated water by Scenedesmus abundans. Chemosphere 47:249–255
Thauer RK (1983) The new nickel enzymes from anaerobic bacteria. Naturwissen Schaften 70:50
Trivedi J, Aila M, Bangwal DP, Kaul S, Garg MO (2015) Algae based biorefinery—how to make sense? Sustain Energy Rev 47:295–307
Van Baalen C, O’Donnell R (1978) Isolation of nickel-dependent blue-green algae. J Gen Microbiol 105:351
Vymazal J (1995) Algae and element cycling in wetlands. CRC Press, Boca Raton, FL, p 689
Walker JB (1953) Inorganic micro nutrient requirements of Chlorella 1- requirements for calcium (or strontium), copper and molybdenum Arch Biochem. Biophys 46:1–11
Wang XL, Zhang L, Han XR, Zhu CJ, Ge M (2002) Effect of nutrient on marine phytoplankton growth—study on mathematic model. Adv Mar Sci 20:96–101
Wong MH, Pak DCH (1992) Removal of copper and nickel by free and immobilized microalgae. Biomed Environ Sci 5(2):99–108 (PMID: 1642794)
Woolhouse HW (1983) Toxicity and tolerance in the responses of plants to metals. In Encyclopedia of plant Physiology, Vol. 12C: Responses to the Chemical and Biological Environment (eds O. L. Lange, P. S. Nobel, C B. Osmond and H. Ziegler): 245 - 300. Springer-Verlag, Berlin
Yuliani G, Mudzakir A, Wulandari AP (2019) Characterization and physiological of chlorophyll extract from Spirulina sp. J Phys Conf 1280(2):022013
Zarrouk C (1966) Contribution à l’étuded’unecyanophycée. Influence de Divers Facteurs Physiques et Chimiques Sur la Croissance et la Photosynthèse de Spirulina maxima. Ph.D. Thesis, Université De Paris, Paris
Zinicovscaia I, Cepoi L, Rudi L, Chiriac T, Grozdov D, Vergel K (2021) Effect of zinc-containing systems on Spirulina platensis: bioaccumulation capacity and biochemical composition. Environ Sci Pollut Res 28:52216–52224
Zinicovscaia I, Rudi L, Valuta A, Cepoi L, Vergel K, Frontasyeva M, Safonov A, Wells M, Grozdov D (2016) Biochemical changes in Nostoc linkia associated with selenium nanoparticles biosynthesis. Ecol Chem Eng S 3(4):559–569
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MK, NEA, HES, and AEZ contributed significantly to the manuscript preparation. MK, NEA, and HES designed the experiment and performed the laboratory analyses. AEZ processed satellite images, generated the spatial distribution maps, and performed the suitability analyses. MK, NEA, HES, and AEZ carried out the statistical analyses and tabulated the study results. MK, NEA, HES, and AEZ wrote the first draft of the manuscript. MK and AEZ revised the final version of the manuscript. MK, NEA, HES, and AEZ read and agreed on the submitted paper.
Corresponding author
Ethics declarations
Ethical approval
Not applied.
Consent to participate
I voluntarily agree to participate in this research study. I understand that even if I agree to participate now, I can withdraw at any time or refuse to answer any question without any consequences of any kind.
Consent for publication
The author warrants that the work has not been published before in any form and is not under consideration by another publisher that the persons listed above are in the proper order and that no author entitled to credit has been omitted, and generally that the authors have the right to make the grants made to the Publisher complete and unencumbered. The author also warrants that the work does not libel anyone, infringe anyone’s copyright, or otherwise violate anyone’s statutory or common law rights.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaamoush, M., El-Agawany, N., Salhin, H.E. et al. Monitoring effect of nickel, copper, and zinc on growth and photosynthetic pigments of Spirulina platensis with suitability investigation in Idku Lake. Environ Sci Pollut Res 29, 78942–78959 (2022). https://doi.org/10.1007/s11356-022-21328-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21328-1