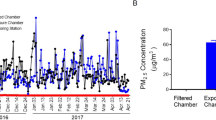
Abstract
Epidemiological studies have demonstrated the association between exposure to fine particulate matter (PM2.5) and the onset of non-alcoholic fatty liver disease (NAFLD). However, the potential biological mechanism is largely unknown. Our study was aimed to explore the impact of PM2.5 on the transcriptome level in the liver of ob/ob mice by atmosphere PM2.5 whole-body dynamic exposure system, and meanwhile preliminarily investigated the effects of metformin intervention in this process. More than three thousand differentially expressed genes (DEGs) was screened out by microarray analysis (p < 0.05, |FC|> 1.5). KEGG pathway enrichment analysis showed that these DEGs were mainly enriched in cancers, infectious diseases, and signal transduction, and the most significant pathways were thyroid hormone signaling pathway, chronic myeloid leukemia and metabolic pathways. Then, 12 hub genes were gained through weighted gene correlation network analysis (WGCNA) and verified by qRT-PCR. The expression of 5 genes in darkslateblue module (cd53, fcer1g, cd68, ctss, laptm5) increased after PM2.5 exposure and decreased after metformin intervention. They were related to insulin resistance, glucose and lipid metabolism and other liver metabolism, and also neurodegenerative diseases. This study provided valuable clues and possible protective measures to the liver damage in ob/ob mice caused by PM2.5 exposure, and further research is needed to explore the related mechanism in detail.
Graphical abstract







Similar content being viewed by others
Data availability
All microarray data is MIAME compliant and the raw data has been deposited in NCBIs Gene Expression Omnibus (NCBIs GEO ID: GSE186900, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186900).
Abbreviations
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Onotology
- STC-GO:
-
Series test of cluster of gene ontology
- DEGs:
-
Differentially expressed genes
- WGCNA:
-
Weighted correlation network analysis
- qRT-PCR:
-
Real-time quantitative polymerase chain reaction
References
Atangwho IJ, Yin KB, Umar MI et al (2014) Vernonia amygdalina simultaneously suppresses gluconeogenesis and potentiates glucose oxidation via the pentose phosphate pathway in streptozotocin-induced diabetic rats. BMC Complement Altern Med [j] 14:426
Bano A, Chaker L, Muka T et al (2020) Thyroid function and the risk of fibrosis of the liver, heart, and lung in humans: a systematic review and meta-analysis. Thyroid [j] 30:806–820
Carvalho H (2021) New WHO global air quality guidelines: more pressure on nations to reduce air pollution levels. Lancet Planet Health [j] 5:e760–e761
Cui S, Sun H, Gu X et al (2015) Gene expression profiling analysis of locus coeruleus in idiopathic Parkinson’s disease by bioinformatics. Neurol Sci [j] 36:97–102
Ehses JA, Lacraz G, Giroix MH et al (2009) IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A [j] 106:13998–14003
Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology [j] 51:679–689
Fan Q, Yang L, Zhang X et al (2018) Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res [j] 37:9
Ghassabian A, Pierotti L, Basterrechea M et al (2019) Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open [j] 2:e1912902
Haberzettl P, Mccracken JP, Bhatnagar A et al (2016) Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol [j] 310:H1423-1438
Hajduch E, Heyes RR, Watt PW et al (2000) Lactate transport in rat adipocytes: identification of monocarboxylate transporter 1 (MCT1) and its modulation during streptozotocin-induced diabetes. FEBS Lett [j] 479:89–92
Han HS, Kang G, Kim JS et al (2016) Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med [j] 48:e218
He L, Sabet A, Djedjos S et al (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell [j] 137:635–646
Hsieh S, Leaderer BP, Feldstein AE et al (2018) Traffic-related air pollution associations with cytokeratin-18, a marker of hepatocellular apoptosis, in an overweight and obese paediatric population. Pediatr Obes [j] 13:342–347
Huang DQ, El-Serag HB, Loomba R (2021) Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol [j] 18:223–238
Huang F, Li X, Wang C et al (2015) PM2.5 Spatiotemporal variations and the relationship with meteorological factors during 2013–2014 in Beijing, China. Plos One [J] 10:e0141642
Jia Z, Wei Y, Li X et al (2018) Exposure to ambient air particles increases the risk of mental disorder: findings from a natural experiment in Beijing. Int J Environ Res Public Health 15(1):160. https://doi.org/10.3390/ijerph15010160
Karagianni P, Talianidis I (2015) Transcription factor networks regulating hepatic fatty acid metabolism. Biochim Biophys Acta [j] 1851:2–8
Kim HJ, Kwon H, Yun JM et al (2020) Association between exposure to ambient air pollution and thyroid function in Korean adults. J Clin Endocrinol Metab 105(8):dgaa338. https://doi.org/10.1210/clinem/dgaa338
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics [j] 9:559
Liang S, Zhao T, Hu H et al (2019) Repeat dose exposure of PM2.5 triggers the disseminated intravascular coagulation (DIC) in SD rats. Sci Total Environ [j] 663:245–253
Liu J, Liang S, Du Z et al (2019) PM(2.5) aggravates the lipid accumulation, mitochondrial damage and apoptosis in macrophage foam cells. Environ Pollut [j] 249:482–490
Liu R, Young MT, Chen JC et al (2016) Ambient air pollution exposures and risk of Parkinson disease. Environ Health Perspect [j] 124:1759–1765
Luo T, Nocon A, Fry J et al (2016) AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes [j] 65:2295–2310
Madiraju AK, Qiu Y, Perry RJ et al (2018) Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med [j] 24:1384–1394
Martínez-Sánchez N, Seoane-Collazo P, Contreras C et al (2017) Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab [j] 26:212-229.e212
Mukherjee A, Agrawal M (2018) A global perspective of fine particulate matter pollution and its health effects. Rev Environ Contam Toxicol [j] 244:5–51
Reyes-Caballero H, Rao X, Sun Q et al (2019) Air pollution-derived particulate matter dysregulates hepatic Krebs cycle, glucose and lipid metabolism in mice. Sci Rep [j] 9:17423
Ritter MJ, Amano I, Hollenberg AN (2020) Thyroid hormone signaling and the liver. Hepatology [j] 72:742–752
Romanque P, Cornejo P, Valdés S et al (2011) Thyroid hormone administration induces rat liver Nrf2 activation: suppression by N-acetylcysteine pretreatment. Thyroid [j] 21:655–662
Shi Q, Zhao L, Xu C et al (2019) High molecular weight hyaluronan suppresses macrophage M1 polarization and enhances IL-10 production in PM(2.5)-induced lung inflammation. Molecules 24(9):1766. https://doi.org/10.3390/molecules24091766
Simon TG, Roelstraete B, Khalili H et al (2021) Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut [j] 70:1375–1382
Sinha RA, Singh BK, Yen PM (2018) Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol [j] 14:259–269
Sun S, Yang Q, Zhou Q et al (2021) Long-term exposure to fine particulate matter and non-alcoholic fatty liver disease: a prospective cohort study. Gut 71(2):443–445. https://doi.org/10.1136/gutjnl-2021-324364
Tian Z, He W, Tang J et al (2020) Identification of important modules and biomarkers in breast cancer based on WGCNA. Onco Targets Ther [j] 13:6805–6817
Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol [j] 64:1147–1161
Vopham T, Bertrand KA, Tamimi RM et al (2018) Ambient PM(2.5) air pollution exposure and hepatocellular carcinoma incidence in the United States. Cancer Causes Control [j] 29:563–572
Wang Y, Li C, Zhang X, et al. (2021) Exposure to PM2.5 aggravates Parkinson’s disease via inhibition of autophagy and mitophagy pathway. Toxicology [J] 456:152770
Weichenthal S, Hoppin J A, Reeves F (2014) Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring) [J] 22:1580–1589
WHO (2021a) WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. https://www.who.int/publications/i/item/9789240034228
WHO (2021b) Ambient (outdoor) air pollution. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
Wu J, Shi Y, Asweto CO et al (2017) Fine particle matters induce DNA damage and G2/M cell cycle arrest in human bronchial epithelial BEAS-2B cells. Environ Sci Pollut Res Int [j] 24:25071–25081
Xu MX, Ge CX, Qin YT et al (2019) Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic Biol Med [j] 130:542–556
Yan R, Ku T, Yue H, et al. (2020) PM(2.5) exposure induces age-dependent hepatic lipid metabolism disorder in female mice. J Environ Sci (China) [J] 89:227–237
Yu H, Liu Y, Li C et al (2020) Bioinformatic analysis of neuroimmune mechanism of neuropathic pain. Biomed Res Int [j] 2020:4516349
Zaitsev AV, Martinov MV, Vitvitsky VM et al (2019) Rat liver folate metabolism can provide an independent functioning of associated metabolic pathways. Sci Rep [j] 9:7657
Zheng Z, Xu X, Zhang X et al (2013) Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol [j] 58:148–154
Zheng Z, Zhang X, Wang J et al (2015) Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol [j] 63:1397–1404
Zhou Y, Zhou B, Pache L et al (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun [j] 10:1523
Funding
This work was supported by the National Natural Science Foundation of China (91943301, 92043301, 81602876). The authors would like to thank Weiping Tang of Cnkingbio biotechnology Co. Ltd. for bioinformatics assistance.
Author information
Authors and Affiliations
Contributions
LL: writing—first draft, software, visualization, and investigation. LT: writing—first draft, conceptualization, and methodology. TL: visualization and investigation. MS: data curation and investigation. JD: investigation and writing—reviewing and editing. YY: supervision, writing—reviewing and editing. ZS: writing—reviewing and editing.
Corresponding authors
Ethics declarations
Ethics approval
This work has received approval for research ethics from the Animal Care and Use Committee of Capital Medical University, which ethical approval number is AEEI-2019–161.
Consent to participate
Not applicable.
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Real time exposure of PM2.5 disturbs the transcriptome level in ob/ob mice liver.
2. PM2.5 affects insulin resistance, glucose, and lipid metabolism in obese fatty liver.
3. Metformin could protect the PM2.5-induced metabolic disturbance in obese fatty liver.
4. WGCNA reveals 12 hub genes as potential biomarkers in PM2.5-induced hepatic injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, L., Tian, L., Li, T. et al. Microarray analysis of mRNA expression profiles in liver of ob/ob mice with real-time atmospheric PM2.5 exposure. Environ Sci Pollut Res 29, 76816–76832 (2022). https://doi.org/10.1007/s11356-022-21088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21088-y