Abstract
Plant leaf meal of some forage trees such as Moringa oleifera has attracted an increasing interest as a good and cheap source of protein. The present in vitro experiment employed the in vitro wireless gas production (GP) technique to evaluate the inclusion of M. oleifera leaves ensiled for 45 days as a replacement for soybean meal in rations. A control basal ration was formulated to contain 17.5% soybean meal as a source of protein. Soybean meal in the control ration was replaced with silage (MOS) at increasing levels of 0 to 100%. Replacing soybean meal with MOS gradually increased (P < 0.001) GP kinetics (asymptotic GP, rate of GP, and lag time of GP). However, soybean meal replacement decreased (P < 0.001) asymptotic methane (CH4) and carbon dioxide (CO2) productions, and rate of CH4 production and increased the lag time of CH4 and CO2 production. Gradual increases (P < 0.001) in the digestibility of dry matter, neutral detergent fiber and acid detergent fiber, ruminal bacteria count, fermentation pH, and the concentrations of ruminal total volatile fatty acids, acetate, and propionate were observed with rations containing MOS. Decreases in the digestibility of crude protein, ruminal protozoal count, and the concentrations of ruminal ammonia-N were observed with MOS rations. It is concluded soybean meal can be completely replaced by MOS with desirable effects on ruminal fermentation.
Similar content being viewed by others
Introduction
Protein feeds are the most important components of animal’s ration. Due to their scarcity and high prices, it is necessary to explore and evaluate unconventional plants, rich in protein, as suitable and viable alternatives to conventional animal protein feeds (Abarghuei and Salem 2021; Özelçam et al. 2021). Protein-rich plant leaf meals, with a good amino acid profile and low prices, can be used as suitable alternatives (Kholif et al. 2016). In their review, Kronqvist et al. (2021) noted that feeding foliage to goats increased feed intake and average daily weight gain compared with grass-based diets. Additionally, they observed a decreased neutral detergent fiber (NDF) and higher rotein (CP) in the foliage than in the grass. Özelçam et al. (2021) evaluated the nutritive value of ensiled or dried Paulownia spp. leaves and observed a considerable in vitro organic matter digestibility, metabolizable energy value, and rumen fermentation characteristics, making them good feeds for ruminants. Moringa oleifera is an another excellent example of protein leaf meal and secondary metabolites that showed good results as an alternative for conventional protein feeds in ruminants (Kholif et al. 2016).
M. oleifera, an indigenous tree native to the Himalaya, has been widely distributed almost worldwide. It can be grown successfully in a variety of conditions such as hot, humid, dry, and subtropical tropics, with multiple time harvests. The silage of M. oleifera leaves contains about 30% CP (dry matter (DM) basis) of the whole plant, with a high concentration of bypass protein and an adequate amino acid profile (Ebeid et al. 2020a). In vitro evaluation (Ebeid et al. 2020a), and experiments on lactating goats (Kholif et al. 2018), sheep (Kewan et al. 2019), and calves (Abdel-Raheem and Hassan 2021), promising results, including feed utilization, milk production and composition, and milk fatty acids profile, were reported. Kholif et al. (2016) evaluated fresh or ensiled leaves of M. oleifera as a replacement of sesame meal at different levels in the diet of lactating goats and observed improved feed digestion, ruminal fermentation, and milk production and composition. Using in sacco fermentation, Ebeid et al. (2020a) evaluated M. oleifera leaves and seeds and observed that leaves showed better nutritive value compared to seeds. They observed that M. oleifera leaves had a high DM disappearance and effective degradability. Additionally, Ebeid et al. (2020b) observed that M. oleifera increased microbial protein and propionate concentration and decreased ruminal protozoal and methanogen counts.
It is well documented that secondary metabolites in some plant species can mitigate methane (CH4) production from ruminal fermentation (Parra-Garcia et al. 2019; Kholif and Olafadehan 2021). M. oleifera is a plant rich in secondary metabolites including tannins, saponins, and many other phenolic compounds (Kholif et al. 2016). Such compounds have the ability to suppress methanogens such as Methanobrevibacter spp., Methanomicrobium spp., Methanobacterium spp., Methanosarcina spp., and methanogenic archaea (Kholif and Olafadehan 2021). Saponins reduce CH4 production via inhibition of ruminal protozoa (Ebeid et al. 2020b). Tannins had the potential to reduce CH4 production by about 50% (Goel and Makkar 2012) due to their antimicrobial properties, which inhibit some ruminal CH4-producing bacteria and protozoa by binding dietary proteins and microbial cell enzymes (Bodas et al. 2012).
The aim of this experiment was to evaluate the potential use of green, cheap, and readily available foliage (i.e., M. oleifera) as a replacement of soybean meal, the costliest feedstuff in livestock ration formulation, to increase farmers’ gain and reduce environmental pollution. Therefore, this experiment evaluated the effects of replacing soybean meal with different levels (0 to 100%) of M. oleifera silage (MOS) on in vitro gas production (GP), biogases production, nutrient degradability, and ruminal fermentation. We hypothesized that the relatively high CP content of M. oleifera leave silage (24.2% DM basis), good amino acids profile, and moderate secondary metabolites would improve ruminal fermentation and decrease biogas production when used as a replacement for soybean meal. Additionally, it was hypothesized that the low CP degradability of MOS (high bypass protein) would match the high CP concentration in soybean meal (high CP degradability and low bypass protein).
Materials and methods
M. oleifera cultivation
M. oleifera seeds were planted at a density of 100,000–150,000 seeds per ha (Kholif et al. 2016). The field was irrigated (900 m3 water/ha) twice a week without fertilizer. The first cutting was carried out after 65 days at about 65–70 cm height. Due to the high moisture content, the first cut was not used. The second cut of M. oleifera was taken after 45 days and used for the in vitro evaluation. After cutting, leaves and young twigs were left in the field for an hour then cut into small pieces, and molasses were added at 5% fresh weight. The materials were then manually packed into polythene bags (40 × 70 cm) and compressed to create anaerobic conditions. The bags were hermetically sealed and stored in dry conditions for 45 days. A small amount of the ensiled material was dried and kept for the in vitro evaluation and chemical analysis.
Experimental rations
Control basal ration, containing (per kg DM) berseem hay and a concentrate feed mixture at 1:1 DM basis, was formulated. The concentrate feed mixture contained (DM basis) 400 g corn grains, 200 g wheat bran, 350 g of soybean meal, and 50 g vitamins/minerals mixture. Ten rations, in which soybean meal was replaced with dried MOS at 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100%, were formulated and used for the in vitro incubation. Feed samples (ingredients and rations) were dried at 60 °C in a forced-air oven for 48 h then mixed and ground to pass a 1-mm screen in a mill. The ground samples were stored for chemical analyses. The ingredient and chemical composition of the formulated rations used as substrates is presented in Table 1.
Feed analysis
Following the official methods of AOAC (2005), samples of MOS, feed ingredients, and rations were analyzed for N content using Kjeldahl method. Ether extract (EE) content was measured using petroleum ether in a Soxhlet extractor. Ash content was measured after burning the samples in a muffle furnace at 550 °C. Concentrations of non-structural carbohydrate (NSC = 1000–NDF–CP–EE–ash) and organic matter (OM = 1000–ash) were calculated. NDF content was determined according to Van Soest et al. (1991) with the use of sodium sulfite and alpha amylase. ADF concentration was analyzed and expressed exclusive of residual ash as described in the AOAC (2005) official method.
Concentrations of tannin (Makkar 2003), total phenolic (Meier et al. 1988), and saponins (Farajzadeh et al. 2020) were determined in MOS. Additionally, the quality of MOS was assessed by measuring pH, volatile fatty acids (VFA), and N-ammonia as detailed by Kholif et al. (2022). Aflatoxin (AF1) concentration was measured in MOS using a fluorometer (Series-4, VICAM, USA), based on the methods described by AOAC (2005).
In vitro fermentation and biodegradation
As previously detailed by Ebeid et al. (2022), the in vitro ruminal fermentation was performed using 250-mL bottles (ANKOMRF Gas Production System) fitted with an automatic wireless GP module (Ankom Technology, Macedon, NY, USA) and pressure sensors. An incubation medium (buffer, macromineral, micromineral, and resarzurin solutions) was prepared (Goering and Van Soest 1970) in a volumetric flask at 39 °C. To remove O2 from the buffer solution, a reduction agent (sodium sulfide solution) was added (2 mL) to the buffer shortly before rumen fluid addition. In each incubation module (250 mL bottle), 20 mL of ruminal inoculum was mixed with 80 ml of buffer, while allowing a head space of 150 mL.
Rumen inoculum was collected from the rumen of three slaughtered Barki rams (51.7 kg body weight) in a local slaughterhouse. The rams were ad libitum fed a diet containing concentrates, berseem hay, and rice straw at 500:400:100 (DM basis), with free access to water. The rumen contents were individually collected from each ram in a thermos and maintained at 39 °C until transported to the laboratory where a little carbon dioxide (CO2) was added. Upon arrival to laboratory, ruminal fluid was filtered through four-layered cheesecloth and then the particulate materials were squeezed to obtain microbes attached to feed particles. Ruminal fluids from the rams were mixed before use.
The control and formulated rations were tested in three bottles (analytical replicates) and two incubation runs in 2 successive weeks with 2 bottles containing inoculum and buffer but no feed (blanks) (11 treatments × 3 replicates × 2 incubation runs + 2 blank bottles). A 1 g ± 10 mg sample for each ration was weighed into filter bags (ANKOM F57; Ankom Technology, Macedon, NY, USA) and the bags were put into 250-mL bottles.
Accumulated gas pressure converted to volume (ml) at standard pressure and temperature was measured. The average GP in the empty bottles (empty corrected GP) was subtracted to obtain the net GP after 2, 4, 6, 8, 10, 12, 16, 20, 24, 36, 48, 72, and 96 h of starting the incubation. At each incubation time, 5 mL of gas was taken from the sampling hole of each module and injected into a Gas-Pro detector (CROWCON Model Tetra3 Gas Analyzer, Abingdon, UK) to measure CH4 and CO2 concentrations in the total gas (Kholif et al. 2022).
After 96 h of incubation, bottles were swirled in ice for 5 min to terminate the incubation, and the pH was measured immediately. The filter bags were removed from the bottles and dried in a forced air oven at 55 °C for 48 h. The degradations of DM, NDF, and ADF were calculated by difference between the initial (substrates) and final (residues) weights of the dried substrate DM or NDF or ADF, respectively.
At the end of incubation, 5 mL of the fluid samples was collected from each bottle in glass tubes for measuring ammonia-N and total and individual VFA concentrations as detailed by Kholif et al. (2022). Individual VFA were measured using a chromatography after processing 1.6 mL of strained rumen fluid with 0.4 mL of a solution containing 250 g of metaphosphoric acid as described previously using a gas chromatograph (GC, Thermo fisher scientific, Inc., TRACE1300, Rodano, Milan, Italy). The GC was fitted with an AS3800 autosampler and equipped with a capillary column HP-FFAP (19091F-112; 0.320 mm o.d., 0.50 μm i.d., and 25 m length; J & W Agilent Technologies Inc., Palo Alto, CA, USA). A mixture of known concentrations of individual VFA was used as an external standard (Sigma Chemie GmbH, Steinheim, Germany) to calibrate the integrator (Kholif et al. 2022).
Samples of fermented fluid (4 mL) were individually mixed with 4 mL of methyl green-formalin-saline solution and stored in a refrigerator at 4 °C until analysis of bacterial and protozoal count following the procedure described by Dehority (1993). Total bacteria concentration was determined using a Petroff-Hausser counting chamber (Hausser Scientific®, 3900, Horsham, PA) and a phase contrast microscope at a magnification of 100 × .
Gas production kinetics and statistical analyses
As previously detailed by Kholif et al. (2022), the kinetics of total gas, CO2, and CH4 production (mL/g DM) were estimated using the NLIN procedure of SAS (Online Version 9.4, SAS Inst., Inc., Cary, NC) according to France et al. (2000) model.
Data were analyzed using the GLM procedure of SAS (SAS Inst. Inc. Cary, NC, USA) in a complete randomized design using the model: Yij = μ + Ri + εij where Yij is the observation, μ is the population mean, Ri is the replacement level effect, and εij is the residual error. Data of each of the two runs of the same sample of the substrate were averaged prior to statistical analysis. Mean values of each individual run were used as the experimental unit. Linear and quadratic contrasts were used to examine responses to increasing replacement levels.
Results
Nutrient concentration
Replacing soybean meal with MOS gradually decreased the concentration of CP and increased NDF and ADF concentrations in rations (Table 1).
Biogases production
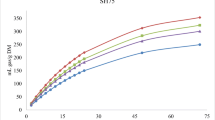
Figures 1, 2, and 3 show the in vitro rumen total gas, CH4, and CO2 production, respectively, from rations containing different levels of MOS replacing soybean meal at different incubation hours. Replacing soybean with MOS gradually increased (linear and quadratic effects, P < 0.001) the asymptotic GP and rate of GP while the lag time of GP was linearly (P < 0.01) increased (Table 2).
Conversely, replacing soybean with MOS gradually decreased (linear effect, P < 0.001) the kinetic of biogases production (the asymptotic and the rates of CH4 and CO2 productions) and increased the lag time of CH4 and CO2 production, without affecting the rate of CO2 production (Table 2).
Nutrient degradability and ruminal fermentation
Replacing soybean with MOS gradually increased (linear effect, P < 0.001) the digestibility of DM, NDF, and ADF, while it decreased the digestibility of CP (linear effect, P < 0.001) (Table 3).
A gradual increase in ruminal bacterial count (linear and quadratic effects, P < 0.01) and a gradual decrease in ruminal protozoal count (linear effect, P < 0.001) were observed when soybean meal was replaced with MOS (Table 3). Linear increases in fermentation pH (P < 0.001), and the concentrations of ruminal total VFA, acetate, and propionate, and linear decreases (P < 0.001) in the concentrations of ruminal ammonia-N were observed when MOS replaced soybean meal in the rations.
Discussion
Nutrient concentration
Replacing soybean meal with MOS gradually decreased dietary CP because MOS contained less protein (242 vs. 438 g/kg DM, respectively) and higher fiber than soybean meal (318 g NDF and 291 g ADF vs. 189 g NDF and 120 g ADF/kg DM, respectively). Chemical composition and nutrient concentration are among the major factors affecting the nutritive value of feeds and ruminal fermentation (Kholif et al. 2017).
Biogases production
Ration containing MOS increased GP and rate of GP, indicating that increased fiber and decreased CP concentrations may be the main reasons for the increased gas production and lag time of GP. The simultaneous increase in total GP and lag time of GP indicates that most of the produced gas was lately produced as a result of late fiber fermentability and fiber is one of the main reasons for increased GP. Increased fiber and decreased CP contents in MOS diets may be another reason. Fermentation of protein gives a relatively small amount of gas compared to fermentation of carbohydrates (Makkar et al. 1995). This observation is not consistent with the observations of Kholif et al. (2017) who noted that increased fiber concentration in rations reduced total GP and increased lag time of GP. Moreover, this is inconsistent with the observations of Soliva et al. (2005) who found that replacing soybean meal and rapeseed meal with M. oleifera leaves decreased in vitro GP. The observed increased DM, NDF, and ADF digestibility and bacterial number with MOS rations may explain the increased GP as shown by Getachew et al. (2004) who confirmed a strong correlation between DM digestibility and total GP. The presence of small amounts of secondary metabolites in MOS, even at high inclusion levels, may be another reason. Generally, suitable (e.g., low and moderate) levels of plant secondary metabolites improve the activity of ruminal bacteria to degrade low and moderate concentrations of plant secondary metabolites (e.g., phenolic compounds and tannins) and utilize them as energy sources to digest feed and produce gases (Kholif and Olafadehan 2021).
Lowered production of CH4 and CO2 and rate of CH4 production with rations containing MOS is desirable from the environmental point of view. Moreover, increased lag time of CH4 and CO2 production with rations containing MOS was parallel with the result of increased GP. The secondary metabolites (e.g., tannins and phenolics) in MOS (Kholif and Olafadehan 2021) and variation in the chemical composition of the treatments (Soltan et al. 2021) may be responsible for these effects. In their experiments, Kholif et al. (2017) showed that chemical composition of incubated substrates affected in vitro production of CH4 and CO2 due to its effect on nutrient availability and microbial activity in the rumen. Secondary metabolites possess antimicrobial and protozoal properties which abate CH4 production (Kholif and Olafadehan 2021). Additionally, secondary metabolites affect ruminal cellulolytic bacteria (Patra and Saxena 2009) and reduce the formation of gases required for methanogenesis (i.e., CO2 and H2) (Goel and Makkar 2012). Bodas et al. (2012) noted that secondary metabolites in plants inhibit ruminal CH4-producing bacteria and decrease the concentrations of available H2 for methanogensis. Goel and Makkar (2012) observed a 50% reduction in the production of CH4 as a result of tannins and phenolic compound administration. The presence of α-linolenic acid in MOS (Ebeid et al. 2020b) may be another reason for decreased CH4 production (MacHmüller et al. 2000). Similar results were observed by Soliva et al. (2005) when they replaced soybean meal with M. oleifera leaves. The decrease in the protozoal count with MOS rations may be another reason for the lowered CH4 production (Bodas et al. 2012). Bodas et al. (2012) concluded that plant secondary metabolites decreased methanogenesis by approximately 8 to 14% under continuous culture conditions.
Nutrient degradability and ruminal fermentation
Increased fiber and decreased CP in rations did not show any negative effect on nutrient digestibility. The increased digestibility of DM, NDF, and ADF with rations containing MOS may be related to the increased bacterial numbers. As previously mentioned, secondary metabolites and antioxidant properties present in M. oleifera can stimulate ruminal fibrolytic microbe activities and growth (Morgavi et al. 2000; Singla et al. 2021), resulting in faster degradation rate and extent of substrates (Kholif and Olafadehan 2021). Analysis of MOS for tannins and saponins showed that their concentrations (19 and 64 mg/g DM, respectively) were less than the critical levels which impair ruminal fermentation and feed digestibility (Frutos et al. 2004); Kholif and Olafadehan (2021). Ruminal microflora degrades and utilizes secondary metabolites at low and moderate levels and use them as energy sources without affecting ruminal fermentation (Frutos et al. 2004). Ebeid et al. (2020a) evaluated the degradability of nutrients in M. oleifera leaves and seeds and observed improved degradability of the leaves relative to the seeds.
The decrease in CP digestibility with replacing soybean meal by MOS may be a result of the presence of tannins and other phenolic compounds in the leaves of M. oleifera which can bind protein and decrease its ruminal degradation by ruminal microbes (Frutos et al. 2004; Kholif and Olafadehan 2021). Basha et al. (2014) observed a positive correlation between CP content and CP disappearance rate and a negative correlation between tannin concentration and CP disappearance. Additionally, the relatively high fiber content of MOS could bind N and decrease its availability to rumen microorganisms (Kendall et al. 1991; Ebeid et al. 2020a). This is a favorable effect from the nutritional point of view, as lower CP degradability indicates greater bypass protein that can be utilized in the duodenum (Barry and Manley 1986; Kumar et al. 1995). Ebeid et al. (2020a) evaluated the CP fermentation kinetics of M. oleifera leaves and seeds and observed a low effective degradability and high undegradable protein as well as a high intestinal CP digestibility.
It was expected that increasing MOS levels in the ration would decrease ruminal bacterial count, due to the antimicrobial effects of secondary metabolites, but this was not observed in the present experiment because rations containing MOS increased total ruminal bacterial counts. These results confirm our previous assertion that secondary metabolites in MOS are within acceptable ranges for ruminal bacterial activities. In their reviews and as previously noted, Frutos et al. (2004) and Kholif and Olafadehan (2021) reported high ability of ruminal microflora to utilize secondary metabolites as energy sources. The decreased ruminal protozoa with rations containing MOS partially possibly explains the reason for the high ruminal bacterial counts. Decreased protozoal population often decreases bacterial engulf, as ruminal protozoa are the main predators of bacteria in the rumen (Mathieu et al. 1996). The decrease in ruminal protozoal count with MOS may be related to the secondary metabolites in MOS. The presence of saponins and other plant secondary metabolites was reported to reduce methanogenic archaea and protozoa in rumen (Nowak et al. 2016; Kholif and Olafadehan 2021). Tannins were reported to have a strong defaunating effect, but the mode of action of this process is not clear (Bhatta et al. 2009). As previously noted, the low protozoal count resulted in decreased CH4 production.
The increased fermentation pH with MOS is a desirable effect because the activity of ruminal bacteria depends mainly on ruminal pH. In the present experiment, ruminal pH values were above the level recommended by Ryle and Ørskov (1990) for maximum ruminal bacterial activities, especially fiber digestion. Increased fiber contents of MOS rations must have been accompanied by increased salivation which invariably buffered the ruminal pH (Zebeli et al. 2012; Olafadehan et al. 2020). However, replacing soybean with MOS decreased the concentration of ruminal ammonia-N, with values being above the recommended level for maximum ruminal bacterial activities (Satter and Slyter 1974). The reduction of CP digestibility in MOS rations may be the main reason for the decreased ammonia-N concentration (Kholif et al. 2016). Additionally, the secondary metabolites in MOS, especially tannins, could reduce protein degradability in the rumen because tannins can bind dietary protein and protect it from ruminal degradation (Frutos et al. 2004). The decrease in total ruminal protozoal count cannot also be ignored as a reason for reduced ammonia-N concentration, because ruminal protozoa play an important role in protein degradation (Jouany 1996). Another possible reason for decreased ammonia-N is the inhibition of ammonia-producing bacteria by plant secondary metabolites (Newbold et al. 2004). The reduced ammonia-N concentration of MOS rations confirms the decreased rate and extent of proteolysis of CP of MOS compared to soybean meal. Earlier reports by Olafadehan and Adebayo (2016) attributed increased ammonia-N concentration of urea-ammoniated threshed sorghum tops vs. dried brewers’ grains to enhanced rate and degree of ruminal proteolysis of N from urea-ammoniated threshed sorghum tops.
The increased concentrations of total VFA, acetate, and propionate with MOS containing rations indicate improved ruminal fermentation, activity of ruminal bacteria, and feed digestion. In their review articles, Bodas et al. (2012) and Kholif and Olafadehan (2021) stated that plants containing secondary metabolites increased production of ruminal propionate and sometimes acetate as a result of improved digestion of structural and nonstructural carbohydrates.
Conclusion
Soybean meal can be completely replaced with M. oleifera leave silage to increase gas production and decrease methane and carbon dioxide production, improve fiber digestibility, and decrease crude protein digestibility. Additionally, M. oleifera leave silage increased the concentrations of ruminal bacteria, total volatile fatty acids, acetate, and propionate and decreased the count of ruminal protozoa. Replacing soybean meal with M. oleifera leave silage up to 100% could be a valuable means of sustainable improvement of the environmental conditions through mitigation of methane and carbon dioxide emissions. Further studies are needed to establish the efficacy of replacement of soybean meal with M. oleifera leave silage in in vivo trials for production performance and greenhouse gas mitigation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abarghuei MJ, Salem AZM (2021) Sustainable impact of pulp and leaves of Glycyrrhiza glabra to enhance ruminal biofermentability, protozoa population, and biogas production in sheep. Environ Sci Pollut Res 28:33371–33381. https://doi.org/10.1007/s11356-021-12968-w
Abdel-Raheem SM, Hassan EH (2021) Effects of dietary inclusion of Moringa oleifera leaf meal on nutrient digestibility, rumen fermentation, ruminal enzyme activities and growth performance of buffalo calves. Saudi J Biol Sci 28:4430–4436. https://doi.org/10.1016/j.sjbs.2021.04.037
AOAC (2005) Official methods of analysis of AOAC international, 16th edn. AOAC International, Washington DC
Barry TN, Manley TR (1986) Interrelationships between the concentrations of total condensed tannin, free condensed tannin and lignin in Lotus sp. and their possible consequences in ruminant nutrition. J Sci Food Agric 37:248–254. https://doi.org/10.1002/jsfa.2740370309
Basha NAD, Peter SF, Ahmed MA, Nsahlai IV (2014) Effects of season, browse species and polyethylene glycol addition on in vitro degradability of forages in the sub-humid subtropical savannah, South Africa. J Agric Ext Rural Dev 6:153–161. https://doi.org/10.5897/JAERD2014.0597
Bhatta R, Uyeno Y, Tajima K, Takenaka A, Yabumoto Y, Nonaka I, Enishi O, Kurihara M (2009) Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J Dairy Sci 92:5512–5522. https://doi.org/10.3168/jds.2008-1441
Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, López S (2012) Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim Feed Sci Technol 176:78–93. https://doi.org/10.1016/j.anifeedsci.2012.07.010
Dehority BA (1993) Laboratory manual for classification and morphology of rumen ciliate protozoa, 1st edn. CRC Press, Boca Raton, FL, USA
Ebeid HM, Kholif AE, Chrenkova M, Anele UY (2020a) Ruminal fermentation kinetics of Moringa oleifera leaf and seed as protein feeds in dairy cow diets: in sacco degradability and protein and fiber fractions assessed by the CNCPS method. Agrofor Syst 94:905–915. https://doi.org/10.1007/s10457-019-00456-7
Ebeid HM, Kholif AE, El-Bordeny N, Chrenkova M, Mlynekova Z, Hansen HH (2022) Nutritive value of quinoa (Chenopodium quinoa) as a feed for ruminants: in sacco degradability and invitro gas production. Environ Sci Pollut Res 29: 35241-35252. https://doi.org/10.1007/s11356-022-18698-x
Ebeid HM, Mengwei L, Kholif AE, Hassan F, Lijuan P, Xin L, Chengjian Y (2020b) Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr Microbiol 77:1271–1282. https://doi.org/10.1007/s00284-020-01935-2
Farajzadeh Z, Shakerian A, Rahimi E, Bagheri M (2020) Chemical, antioxidant, total phenolic and flavonoid components and antimicrobial effects of different species of quinoa seeds. Egypt J Vet Sci 51:43–54. https://doi.org/10.21608/ejvs.2019.17122.1098
France J, Dijkstra J, Dhanoa MS, Lopez S, Bannink A (2000) Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr 83:143–150. https://doi.org/10.1017/S0007114500000180
Frutos P, Hervás G, Giráldez FJ, Mantecón AR (2004) Review. Tannins and ruminant nutrition. Spanish J Agric Res 2:191. https://doi.org/10.5424/sjar/2004022-73
Getachew G, Robinson PH, DePeters EJ, Taylor SJ (2004) Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim Feed Sci Technol 111:57–71. https://doi.org/10.1016/S0377-8401(03)00217-7
Goel G, Makkar HPS (2012) Methane mitigation from ruminants using tannins and saponins. Trop Anim Health Prod 44:729–739. https://doi.org/10.1007/s11250-011-9966-2
Goering HK, Van Soest PJ (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications). No. 379. US Agricultural Research Service, Washington, USA
Jouany JP (1996) Effect of rumen protozoa on nitrogen utilization by ruminants. J Nutr 126:1335–1346. https://doi.org/10.1093/jn/126.suppl_4.1335s
Kendall EM, Ingalls JR, Boila RJ (1991) Variability in the rumen degradability and postruminal digestion of the dry matter, nitrogen and amino acids of canola meal. Can J Anim Sci 71:739–754. https://doi.org/10.4141/cjas91-089
Kewan KZ, Salem FA, Salem AZM, Abdou AR, El-Sayed HM, Eisa SS, Zaki EA, Odongo NE (2019) Nutritive utilization of Moringa oleifera tree stalks treated with fungi and yeast to replace clover hay in growing lambs. Agrofor Syst 93:161–173. https://doi.org/10.1007/s10457-017-0158-6
Kholif AE, Elghandour MMY, Salem AZM, Barbabosa A, Márquez O, Odongo NE (2017) The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J Agric Sci 155:494–507. https://doi.org/10.1017/S0021859616000812
Kholif AE, Gouda GA, Olafadehan OA, Abdo MM (2018) Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal 12:964–972. https://doi.org/10.1017/S1751731117002336
Kholif AE, Gouda GA, Patra AK (2022) The sustainable mitigation of in vitro ruminal biogas emissions by ensiling date palm leaves and rice straw with lactic acid bacteria and Pleurotus ostreatus for cleaner livestock production. J Appl Microbiol 132: 2925-2939. https://doi.org/10.1111/jam.15432
Kholif AE, Morsy TA, Gouda GA, Anele UY, Galyean ML (2016) Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim Feed Sci Technol 217:45–55. https://doi.org/10.1016/j.anifeedsci.2016.04.012
Kholif AE, Olafadehan OA (2021) Essential oils and phytogenic feed additives in ruminant diet: chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem Rev 20:1087–1108. https://doi.org/10.1007/s11101-021-09739-3
Kronqvist C, Kongmanila D, Wredle E (2021) Effects of replacing grass with foliage on growth rate and feed intake in goats—a systematic review and meta-analysis. Animals 11:3163. https://doi.org/10.3390/ani11113163
Kumar R, D’mello JPF, Devendra C (1995) Anti-nutritional factors in forage legumes. In: D’Mello FJP, Devendra C (eds) Tropical legumes in animal nutrition. CAB International, Wallingford; UK, pp 95–133
MacHmüller A, Ossowski DA, Kreuzer M (2000) Comparative evaluation of the effects of coconut oil, oilseeds and crystalline fat on methane release, digestion and energy balance in lambs. Anim Feed Sci Technol 85:41–60. https://doi.org/10.1016/S0377-8401(00)00126-7
Makkar HPS (2003) Quantification of tannins in tree and shrub foliage. Springer, Netherlands, Dordrecht
Makkar HPS, Blümmel M, Becker K (1995) Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br J Nutr 73:897–913. https://doi.org/10.1079/BJN19950095
Mathieu F, Jouany JP, Sénaud J, Bohatier J, Bertin G, Mercier M (1996) The effect of Saccharomyces cerevisiae and Aspergillus oryzae on fermentations in the rumen of faunated and defaunated sheep; protozoal and probiotic interactions. Reprod Nutr Dev 36:271–287. https://doi.org/10.1051/rnd:19960305
Meier B, Julkunen-Tiitto R, Tahvanainen J, Sticher O (1988) Comparative high-performance liquid and gas-liquid chromatographic determination of phenolic glucosides in salicaceae species. J Chromatogr A 442:175–186. https://doi.org/10.1016/S0021-9673(00)94467-4
Morgavi DP, Newbold CJ, Beever DE, Wallace RJ (2000) Stability and stabilization of potential feed additive enzymes in rumen fluid. Enzyme Microb Technol 26:171–177. https://doi.org/10.1016/S0141-0229(99)00133-7
Newbold CJ, McIntosh FM, Williams P, Losa R, Wallace RJ (2004) Effects of a specific blend of essential oil compounds on rumen fermentation. Anim Feed Sci Technol 114:105–112. https://doi.org/10.1016/j.anifeedsci.2003.12.006
Nowak V, Du J, Charrondière UR (2016) Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem 193:47–54. https://doi.org/10.1016/j.foodchem.2015.02.111
Olafadehan OA, Adebayo OF (2016) Nutritional evaluation of ammoniated ensiled threshed sorghum top as a feed for goats. Trop Anim Health Prod 48:785–791. https://doi.org/10.1007/s11250-016-1027-4
Olafadehan OA, Okunade SA, Njidda AA, Kholif AE, Kolo SG, Alagbe JO (2020) Concentrate replacement with Daniellia oliveri foliage in goat diets. Trop Anim Health Prod 52:227–233. https://doi.org/10.1007/s11250-019-02002-0
Özelçam H, İpçak HH, Özüretmen S, Canbolat Ö (2021) Feed value of dried and ensiled paulownia (Paulownia spp.) leaves and their relationship to rumen fermentation, in vitro digestibility, and gas production characteristics. Rev Bras Zootec, 50:e20210057. https://doi.org/10.37496/RBZ5020210057
Parra-Garcia A, Elghandour MMY, Greiner R, Barbabosa-Pliego A, Camacho-Diaz LM, Salem AZM (2019) Effects of Moringa oleifera leaf extract on ruminal methane and carbon dioxide production and fermentation kinetics in a steer model. Environ Sci Pollut Res 26:15333–15344. https://doi.org/10.1007/s11356-019-04963-z
Patra AK, Saxena J (2009) Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Antonie Van Leeuwenhoek, Int J Gen Mol Microbiol 96:363–375. https://doi.org/10.1007/s10482-009-9364-1
Ryle M, Ørskov ER (1990) Energy nutrition in ruminants. Springer, Netherlands, Dordrecht
Satter LD, Slyter LL (1974) Effect of ammonia concentration on rumen microbial protein production in vitro. Br J Nutr 32:199–208. https://doi.org/10.1079/bjn19740073
Singla A, Hundal JS, Patra AK, Wadhwa M, Nagarajappa V, Malhotra P (2021) Effect of dietary supplementation of Emblica officinalis fruit pomace on methane emission, ruminal fermentation, nutrient utilization, and milk production performance in buffaloes. Environ Sci Pollut Res 28:18120–18133. https://doi.org/10.1007/s11356-020-12008-z
Soliva CR, Kreuzer M, Foidl N, Foidl G, Machmüller A, Hess HD (2005) Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim Feed Sci Technol 118:47–62. https://doi.org/10.1016/j.anifeedsci.2004.10.005
Soltan Y, Abdalla Filho A, Abdalla A, Berenchtein B, Schiavinatto P, Costa C (2021) Replacing maize with low tannin sorghum grains: lamb growth performance, microbial protein synthesis and enteric methane production. Anim Prod Sci 61:1348–1355. https://doi.org/10.1071/AN20605
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Zebeli Q, Aschenbach JR, Tafaj M, Boguhn J, Ametaj BN, Drochner W (2012) Invited review: role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J Dairy Sci 95:1041–1056. https://doi.org/10.3168/jds.2011-4421
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This paper is based upon work supported by the National Research Centre under Grant: 12050401.
Author information
Authors and Affiliations
Contributions
TAM, GAG, and AEK contributed to the study conception and design. TAM, AEK and GAG prepared materials and collected data. AEK prepared the first draft of the manuscript and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morsy, T.A., Gouda, G.A. & Kholif, A.E. In vitro fermentation and production of methane and carbon dioxide from rations containing Moringa oleifera leave silage as a replacement of soybean meal: in vitro assessment. Environ Sci Pollut Res 29, 69743–69752 (2022). https://doi.org/10.1007/s11356-022-20622-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20622-2