Abstract
Extensive studies have shown that doping can enhance the properties of graphene, but the application to real industrial wastewater treatment and theoretical calculations are limited. In this study, the hybrid nanoadsorbent Cu, N co-doped graphene (Cu@NG) was successfully synthesized via green route using carbon rods from waste dry batteries, human urine and copper nitrate, then multiple characterizations, detailed density functional theory (DFT) theoretical calculations and comprehensive actual wastewater tests are performed in environmental applications to investigate the adsorption properties and mechanism. The results showed that Cu@NG surface is mesoporous, decorated with CuO crystals and doped with N atoms. The isotherms and kinetics were simulated by Langmuir and pseudo-second-order models, respectively. The theoretical maximum sorption for MB and CV on Cu@NG is 116.28 mg·g−1 and CV is 86.96 mg·g−1, respectively. Pilot tests with Cu@NG on real textile wastewater showed that COD, BOD and color were removed by 54.2%, 55.2% and 86.4%, respectively. The desorption rate of Cu@NG is approximately above 90% for both MB and CV on Cu@NG after six cycles of treatment. The DFT calculations confirmed the experimental results as MB is more reactive than CV molecules. Besides, interactions have been systematically investigated via topology and natural bond orbital (NBO) analyses. The process mechanism involved mainly electrostatic adsorption, π-π stacking interactions and H-bonding interactions and ion exchange.
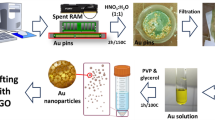
Graphical abstract


















Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeyemo AA, Adeoye IO, Bello OS (2012) Metal organic frameworks as adsorbents for dye adsorption: overview, prospects and future challenges. Toxicol Environ Chem 94:1846–1863
Akinola LK, Umar AM (2015) Adsorption of crystal violet onto adsorbents derived from agricultural wastes: kinetic and equilibrium studies. J Appl Sci Environ Manag 19:279–288
Ali I, Alothman ZA, Alwarthan A (2017) Supra molecular mechanism of the removal of 17-β-estradiol endocrine disturbing pollutant from water on functionalized iron nano particles. J Mol Liq 241:123–129
Alizadeh N, Shariati S, Besharati N (2017) Adsorption of Crystal Violet and Methylene Blue on Azolla and Fig Leaves Modified with Magnetite Iron Oxide Nanoparticles. Int J Environ Res 11:197–206
Allouss D, Essamlali Y, Amadine O, Chakir A, Zahouily M (2019) Response surface methodology for optimization of methylene blue adsorption onto carboxymethyl cellulose-based hydrogel beads: adsorption kinetics, isotherm, thermodynamics and reusability studies. RSC Adv 9:37858–37869
Azari A, Nabizadeh R, Nasseri S, Mahvi AH, Mesdaghinia AR (2020) Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 250:126238
Azha SF, Sellaoui L, Shamsudin MS, Ismail S, Bonilla-Petriciolet A, Ben Lamine A, Erto A (2018) Synthesis and characterization of a novel amphoteric adsorbent coating for anionic and cationic dyes adsorption: Experimental investigation and statistical physics modelling. Chem Eng J 351:221–229
Baig N, Ihsanullah Sajid M, Saleh TA (2019) Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J Environ Manage 244:370–382
Bandi S, Ravuri S, Peshwe DR, Srivastav AK (2019) Graphene from discharged dry cell battery electrodes. J Hazard Mater 366:358–369
Batten T (2013) Rapid Characterization of Large Areas of Graphene Using Raman Spectroscopy. Am Lab 45:15–17
Buhani, Suharso, Aditiya I, Al Kausar R, Sumadi, Rinawati, (2019) Production of a Spirulina sp. algae hybrid with a silica matrix as an effective adsorbent to absorb crystal violet and methylene blue in a solution. Sustain Environ Res 29
Carniato, S., Dufour, G., Luo, Y., Ågren, H., 2002. Ab initiostudy of the Cu2pand3score-level XPS spectra of copper phthalocyanine. Physical Review B 66.
Collivignarelli MC, Abba A, Miino MC, Damiani S (2019) Treatments for color removal from wastewater: State of the art. J Environ Manage 236:727–745
Dai J, Huang T, Tian SQ, Xiao YJ, Yang JH, Zhang N, Wang Y, Zhou ZW (2016) High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for waste water treatment. Materials & Design 107:187–197
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423
Elawwad A, Ragab M, Hamdy A, Husein DZ (2020) Enhancing the performance of microbial desalination cells using δMnO2/graphene nanocomposite as a cathode catalyst. Journal of Water Reuse and Desalination 10:214–226
El-Mahdy AFM, Liu TE, Kuo SW (2020) Direct synthesis of nitrogen-doped mesoporous carbons from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. J Hazard Mater 391:122163
Etaiw SEdH, Shalaby EM, Abd El-Aziz DM, Elzeny I (2021) Ultrasound irradiation synthesis and crystal structure of Co(II) thiocyanate supramolecular complex: Photocatalytic and sonocatalytic degradation of methyl violet 2B dye. Appl Organometallic Chem. 35
Fan S, Wang Y, Wang Z, Tang J, Tang J, Li X (2017) Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J Environ Chem Eng 5:601–611
Fan ZJ, Kai W, Yan J, Wei T, Zhi LJ, Feng J, Ren YM, Song LP, Wei F (2011) Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano 5:191–198
Feng CN, Dong L, Meng JK, Zheng XC, Zheng GP (2019) 3D CuO@nitrogen-graphene aerogel hybrids as anodes for lithium-ion batteries: Gas-liquid interfacial assembly and superior electrochemical performance. J Alloy Compd 784:915–922
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK (2006) Raman Spectrum of Graphene and Graphene Layers. Phys Rev Lett 97:187401
Gautam D, Hooda S (2020) Magnetic Graphene Oxide/Chitin Nanocomposites for Efficient Adsorption of Methylene Blue and Crystal Violet from Aqueous Solutions. J Chem Eng Data 65:4052–4062
Hassanien R, Abed-Elmageed AAI, Husein DZ (2019) Eco-Friendly Approach to Synthesize Selenium Nanoparticles: Photocatalytic Degradation of Sunset Yellow Azo Dye and Anticancer Activity. ChemistrySelect 4:9018–9026
Husein DZ (2019) Facile one-pot synthesis of porous N-doped graphene based NiO composite for parabens removal from wastewater and its reusability. Desalin Water Treat 166:211–221
Husein DZ, Uddin MK, Ansari MO, Ahmed SS (2021) Green synthesis, characterization, application and functionality of nitrogen-doped MgO/graphene nanocomposite. Environ Sci Pollut Res Int 28:28014–28023
Ibrahim M, Mahmoud AA (2009) Computational Notes on the Reactivity of Some Functional Groups. J Comput Theor Nanosci 6:1523–1526
Jamal R, Zhang L, Wang M, Zhao Q, Abdiryim T (2016) Synthesis of poly (3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Prog Nat Sci Mater Int 26:32–40
Jiang Y, Chowdhury S, Balasubramanian R (2017) Nitrogen-doped graphene hydrogels as potential adsorbents and photocatalysts for environmental remediation. Chem Eng J 327:751–763
Jibrael RI, Mohammed MKA (2016) Production of graphene powder by electrochemical exfoliation of graphite electrodes immersed in aqueous solution. Optik 127:6384–6389
Kaniyoor A, Ramaprabhu S (2012) A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Advances 2:032183
Khurana I, Saxena A, Bharti Khurana JM, Rai PK (2017) Removal of Dyes Using Graphene-Based Composites: a Review. Water, Air, & Soil Pollut. 228
Kundu S, Chowdhury IH, Naskar MK (2018) Nitrogen-Doped Nanoporous Carbon Nanospheroids for Selective Dye Adsorption and Pb(II) Ion Removal from Waste Water. ACS Omega 3:9888–9898
Labiadh L, Kamali AR (2019) 3D graphene nanoedges as efficient dye adsorbents with ultra-high thermal regeneration performance. Appl Surf Sci 490:383–394
Lai KC, Lee LY, Hiew BYZ, Thangalazhy-Gopakumar S, Gan S (2019) Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J Environ Sci 79:174–199
Lane JR, Contreras-García J, Piquemal J-P, Miller BJ, Kjaergaard HG (2013) Are Bond Critical Points Really Critical for Hydrogen Bonding? J Chem Theory Comput 9:3263–3266
Li L, Dou Y, Wang L, Luo M, Liang J (2014) One-step synthesis of high-quality N-doped graphene/Fe3O4 hybrid nanocomposite and its improved supercapacitor performances. RSC Adv 4:25658–25665
Lingamdinne LP, Koduru JR, Karri RR (2019) A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J Environ Manage 231:622–634
Liu Y, Wu P, Wu X, Ma C, Luo S, Xu M, Li W, Liu S (2020) Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 210:120649
Liu Y, Zhao Y, Cheng W, Zhang T (2020b) Targeted reclaiming cationic dyes from dyeing wastewater with a dithiocarbamate-functionalized material through selective adsorption and efficient desorption. J Colloid Interface Sci 579:766–777
Madrakian T, Afkhami A, Ahmadi M (2012) Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim Acta Part A Mol Biomol Spectrosc 99:102–109
Maity CK, Hatui G, Verma K, Udayabhanu G, Pathak DD, Nayak GC (2018) Single pot fabrication of N doped reduced GO (N-rGO)/ZnO-CuO nanocomposite as an efficient electrode material for supercapacitor application. Vacuum 157:145–154
Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS (2009) Raman spectroscopy in graphene. Phys Rep 473:51–87
Martins Ferreira EH, Moutinho MVO, Stavale F, Lucchese MM, Capaz RB, Achete CA, Jorio A (2010) Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys Rev B 82:125429
Nekouei F, Nekouei S, Kargarzadeh H (2018) Enhanced adsorption and catalytic oxidation of ciprofloxacin on hierarchical CuS hollow nanospheres@N-doped cellulose nanocrystals hybrid composites: Kinetic and radical generation mechanism studies. Chem Eng J 335:567–578
Ober JA (2018) Mineral commodity summaries 2018. Mineral Commodity Summaries, Reston, VA. 204
Park CM, Kim YM, Kim K-H, Wang D, Su C, Yoon Y (2019) Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini review. Chemosphere 221:392–402
Parvez K, Wu ZS, Li R, Liu X, Graf R, Feng X, Mullen K (2014) Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J Am Chem Soc 136:6083–6091
Peng G, Zhang M, Deng S, Shan D, He Q, Yu G (2018) Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem Eng J 341:361–370
Prabhakar Y, Gupta A, Kaushik A (2021) Microbial degradation of reactive red-35 dye: Upgraded progression through Box-Behnken design modeling and cyclic acclimatization. J Water Process Eng. 40
Ren B, Miao J, Wang S, Xu Y, Zhai Z, Dong X, Liu Z (2021) Nitrogen-rich melamine-based carbon nanosheets prepared via polyvinyl pyrrolidone/ammonia chloride-mediate strategy as an excellent adsorbent for methylene blue adsorption. Adv Powder Technol 32:1774–1784
Rice EW, Baird RB, Eaton AD, Clesceri LS (eds) (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA), Washington, DC
Salame II, Bandosz TJ (2003) Role of surface chemistry in adsorption of phenol on activated carbons. J Colloid Interface Sci 264:307–312
Santha Kumar K, Vellaichamy B, Paulmony T (2019) Visible light active metal-free photocatalysis: N-doped graphene covalently grafted with g-C3N4 for highly robust degradation of methyl orange. Solid State Sciences 94:99–105
Sayilgan E, Kukrer T, Civelekoglu G, Ferella F, Akcil A, Veglio F, Kitis M (2009) A review of technologies for the recovery of metals from spent alkaline and zinc–carbon batteries. Hydrometallurgy 97:158–166
Sodeifian G, Behnood R (2019) Hydrothermal Synthesis of N-Doped GQD/CuO and N-Doped GQD/ZnO Nanophotocatalysts for MB Dye Removal Under Visible Light Irradiation: Evaluation of a New Procedure to Produce N-Doped GQD/ZnO. J Inorg Organomet Polym Mater 30:1266–1280
Song T, Tian W, Qiao K, Zhao J, Chu M, Du Z, Wang L, Xie W, (2021). Adsorption Behaviors of Polycyclic Aromatic Hydrocarbons and Oxygen Derivatives in Wastewater on N-Doped Reduced Graphene Oxide. Sep Purif Technol. 254
Song YL, Li JT, Chen H (2008) Removal of acid brown 348 dye from aqueous solution by ultrasound irradiated exfoliated graphite. Indian J Chem Technol 15:443–448
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329
Staudenmaier L (1898) Verfahren zur Darstellung der Graphitsäure. Ber Dtsch Chem Ges 31:1481–1487
Tajabadi MT, Basirun WJ, Lorestani F, Zakaria R, Baradaran S, Amin YM, Mahmoudian MR, Rezayi M, Sookhakian M (2015) Nitrogen-doped graphene-silver nanodendrites for the non-enzymatic detection of hydrogen peroxide. Electrochim Acta 151:126–133
Van der Maelen JF (2020) Topological Analysis of the Electron Density in the Carbonyl Complexes M(CO)8 (M = Ca, Sr, Ba). Organometallics 39:132–141
Vasanthi DS, Ravichandran K, Kavitha P, Sriram S, Praseetha PK (2020) Combined effect of Cu and N on bandgap modification of ZnO film towards effective visible light responsive photocatalytic dye degradation. Superlattices and Microstructures 145
Wang H, Maiyalagan T, Wang X (2012) Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal 2:781–794
Wang T, Li G, Yang K, Zhang X, Wang K, Cai J, Zheng J (2021) Enhanced ammonium removal on biochar from a new forestry waste by ultrasonic activation: Characteristics, mechanisms and evaluation. Sci Total Environ 778:146295
Wang T, Liu H, Duan C, Xu R, Zhang Z, She D, Zheng J (2020) The Eco-Friendly Biochar and Valuable Bio-Oil from Caragana korshinskii: Pyrolysis Preparation, Characterization, and Adsorption Applications. Materials 13(15):3391
Wang T, Zhang D, Fang K, Zhu W, Peng Q, Xie Z (2021) Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: Adsorption characteristics and mechanisms. J Environ Chem Eng 9(4):105184
Wang T, Zheng J, Liu H, Peng Q, Zhou H, Zhang X (2021c) Adsorption characteristics and mechanisms of Pb(2+) and Cd(2+) by a new agricultural waste-Caragana korshinskii biomass derived biochar. Environ Sci Pollut Res Int 28:13800–13818
Xiang X, Li X, Huang Z, Gao T, Yuan H, Xiao D (2019) Sphere-and-Flake-Structured Cu, N Co-Doped Carbon Catalyst Designed by a Template-Free Method for Robust Oxygen Reduction Reaction. ChemElectroChem 6:1078–1087
Yang S, Liu P, Wang Y, Guo Z, Tan R, Qu L (2020) Electrochemical sensor using poly-(l-cysteine) functionalized CuO nanoneedles/N-doped reduced graphene oxide for detection of lead ions. RSC Adv 10:18526–18532
Yin Q, Liu M, Li Y, Li H, Wen Z (2021) Computational study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 269:129374
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci Rep 6:36143
Zare EN, Motahari A, Sillanpaa M (2018) Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ Res 162:173–195
Zhang D, Wang T, Zhi J, Zheng Q, Chen Q, Zhang C, Li Y (2020a) Utilization of Jujube Biomass to Prepare Biochar by Pyrolysis and Activation: Characterization, Adsorption Characteristics, and Mechanisms for Nitrogen. Materials 13(24):5594
Zhang G, Musgrave CB (2007) Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J Phys Chem A 111:1554–1561
Zhang J, Ren B, Wang P, Wang C (2013) Mechanistic study on adsorption of methylene blue on tea seed powder. Environ Chem 32(8):1539–1545
Zhang T, Wang W, Zhao Y, Bai H, Wen T, Kang S, Song G, Song S, Komarneni S (2021) Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem Eng J. 420
Zhang X, Zhou J, Fan Y, Liu J (2020b) Adsorption of dyes from water by Prunella vulgaris stem and subsequent fungal decolorization. Korean J Chem Eng 37:1445–1452
Zhou M, Tang J, Cheng Q, Xu G, Cui P, Qin LC (2013) Few-layer graphene obtained by electrochemical exfoliation of graphite cathode. Chem Phys Lett 572:61–65
Acknowledgements
The authors are grateful to Dr. Turbasu Sengupta, Virginia Commonwealth University, USA, for his valuable comments.
Funding
Department of Chemistry, Faculty of Science, New Valley University.
Author information
Authors and Affiliations
Contributions
Tongtong Wang was involved in investigation, software, analysis and interpretation of the data, visualization, formal analysis, writing—original draft. Dalal Z. Husein conceived and designed the experiments, DFT calculations and interpretations, resources, validation, characterization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
The authors confirm that the final version of the manuscript has been reviewed, approved and consented for publication by all authors.
Competing interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Waste batteries and human urine are reacted via green route to give the nanocomposite.
• The maximum removal capacity for MB and CV was 116.28 and 86.96 mg·g-1, respectively.
• In reality, COD, BOD and color were removed by 54.2%, 55.2% and 86.4%, respectively.
• Adsorption mechanisms include electrostatic, π- π and H-bonding interactions.
• DFT calculations confirmed the experimental results and composite reactivity.
Rights and permissions
About this article
Cite this article
Wang, T., Husein, D.Z. Novel synthesis of multicomponent porous nano-hybrid composite, theoretical investigation using DFT and dye adsorption applications: disposing of waste with waste. Environ Sci Pollut Res 30, 8928–8955 (2023). https://doi.org/10.1007/s11356-022-20050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20050-2