Abstract
The human coronavirus disease (COVID-19) pandemic is caused by a novel coronavirus; the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2). Natural products, secondary metabolites show positive leads with antiviral and immunotherapy treatments using genomic studies in silico docking. In addition, it includes the action of a mechanism targeting the SARS-CoV-2. In this literature, we aimed to evaluate the antiviral movement of the NT-VRL-1 unique terpene definition to Human coronavirus (HCoV-229E). The effects of 19 hydrolysable tannins on the SARS-CoV-2 were therefore theoretically reviewed and analyzed utilising the molecular operating surroundings for their C-Like protease 3CLpro catalytic dyad residues Angiotensin converting enzyme-2 (MOE 09). Pedunculagin, tercatan, and castalin were detected as interacting strongly with SARS-receptor Cov-2’s binding site and catalytic dyad (Cys145 and His41). SARS-CoV-2 methods of subunit S1 (ACE2) inhibit the interaction of the receiver with the s-protein once a drug molecule is coupled to the s-protein and prevent it from infecting the target cells in alkaloids. Our review strongly demonstrates the evidence that natural compounds and their derivatives can be used against the human coronavirus and serves as an area of research for future perspective.
Similar content being viewed by others
Introduction
The novel Coronavirus (CoV), with a genetic genome of size (27–33 kb), is enclosed, with positive RNA-viruses. Their virion is generally spherical with club-shaped spikes proteins (S protein), which attaches to the surface to produce an appearance of the encased virion like a crown shape (approximate 125 nm in diameter) (Maier et al. 2015). Since the disease started on 12 December 2019, the COVID-19 pandemic caused millions of deaths. Like other RNA viruses, SARS-CoV-2, while adapting to their new human hosts, is prone to genetic evolution with the development of mutations over time, resulting in mutant variants that may have different characteristics than its ancestral strains. Several variants of SARS-CoV-2 have been described during the course of this pandemic, among which only a few are considered variants of concern (VOCs) by the WHO, given their impact on global public health (Zhou et al. 2020). In addition, SARS-CoV and the Middle East respiratory syndrome (MERS) genetic sequences are claimed to be identical to 79.6% (Van Doremalen et al. 2020). In comparison with recorded illnesses, the relative significantly higher contagiously and pandemic potential of SARS-CoV-2 reflects in part a considerable proportion of undocumented infections. Since the first treatment plan for the patients is simply in support of the preceding CoV infection epidemics because no specialized COVID19 vaccination and therapies are available, preventive measures, such as quarantine, are crucial for reduced transmission of viruses in all populations so, few studies were practiced by targeting the natural products and their derivatives for their therapeutic uses and antiviral activities against the SARS-CoV-2, which were proven to be very effective and area of research in the coming days. Significant research has been made on natural products to obtain new antimicrobial agents to offset the growing resistance to the microbes. It is the development of microorganism resistance that is the concern with the currently utilized antiviral medications. Many plants have been explored traditionally for viral infections. Tannins phenols and polyphenols terpenes (e.g., mono-, di- and tri-), have been discovered to be active participants in the SARS-CoV-2 viruses. Bioactive chemicals from the vegetable realm are a valuable and primary source of extract in this case. The contagiousness of the virus makes it difficult to contain and the demand for prophylactic and therapeutic medications makes it necessary to screen the scientific community. At the same time, we should focus on newer approaches such as in-silico docking and genome molecular objectives as helpful methods for the testing of existing natural products [NP] versus entire cell and selective molecular factors. We investigate these possibilities in our review study. In addition to the less addressed population-based characteristics related to NP and SARS-CoV-2, we focused on the biological foundation of virulence. The effects of comorbidities associated with SARS-CoV-2 infection are discussed below.
A large number of ways to explore plant biodiversity for discovery and/or development of natural products (NPs) that can help to cope with the modern tools of NP chemistry (fast identification, deduplication, quick chemical profiling, in silicon screening) and biopsic assessment (high throughput in-vitro trial assays, live infection tests, genomics and host proteomics).
Compared to the present class of medications used for human health, small molecules offer a rich source of new bioactive chemicals and various chemical scaffolds. Plants represent a significant and previously untapped resource in the development, despite their comparative abounds in other pharmacological environments, of antibacterial and antiviral medications, with no such currently licensed medications originating from plant sources. Compounds, mostly from plants and microorganisms, identified in natural sources supply a large number of currently on the market medicinal medicines.
Naturally derived compounds inhibiting SARS-CoV-2 activity
Current development in the fields of phytochemistry, extraction, and refraction technologies for extracts ion and the characterization of lead compounds from complex mixings of NP extracts have to lead to the availability of standardized libraries and extracts with assured resource meant for medicinal extracts. Herbals are used from ancient times for the treatment of various body ailments. These are also used in the treatment of various infectious diseases (Gupta et al. 2021). Although 175 anti-cancer medications authorized between 1940 and 2014, 49% were either NP or derived directly from Natural product’s influence of NPSM, and its structure is significant in other domains, such as anti-infective medicines. A significant majority of the NP plants/drugs were first created with microorganisms and/or microbial interactions with the host.
Current techniques for the development of NP anti-virus medicines are based largely on modest scales of random NP extract and compound screenings. An unrivalled supply of NP for the new antiviral drug discovery paradigm is the current NP depot from crop plants, the marine, and the microbial. To ensure that small important components should not be overlooked in biological screening because of low concentration, extracts with a high antiviral activity must be split up and extracting should also take place.
Naturally derived compounds: alkaloids
Natural compounds containing nitrogen that at least have one heteroatom in the heterocyclic ring structure have been found in alkaloids and are responsible for their basic characteristics and different physiological features. The bioplastic method is that the alkaloids are broadly separated into (1) true alkaloid nitrogen-based heterocyclic compounds formed directly from the amino acids; (2) proto alkaloids derived from the amino acids, which lack the mobility of nitrogen in their heterocyclic system. More than 8000 identified natural chemicals now come into the alkaloid class (Kishimoto et al. 2016).
As a valuable source of pharmaceutical drugs, the class of alkaloids natural compounds has been of great importance for humankind because they demonstrate various bioactivities with a relatively low dose. Alkaloids are therefore often used for drug finding as lead molecules. Technology, especially sequencing from next-generation, allowed us to readily examine the main plan for the creation of alkaloids: genes involved for alkaloid biosynthesis. In the last 10 years, however, novel sequencing of DNA has been developed. Similar chemical structure or biological features to plant alkaloids, including synthetic substances like procaine, may be referred to as alkaloids.
Alkaloids that cover the wide-ranging part of these metabolites are produced in the plant kingdom, around 25% by Gymnosperms and Angiosperms. In other plants of other families, such as the Apocynaceae (Martin et al. 2020), the Asteraceae (Chen et al. 2018b), the Papaveraceae (Yu et al. 2014b), the Routaceae (Ferreira et al. 2016), Solenaceae (Heinig and Aharoni 2014), Erythroxylaceae (Oliveira et al. 2010), and the Fabaceae (He et al. 2019), alkaloids can also be used in plants of various plant families. Several biological actions have been described since this class of natural products was discovered with alkaloids such as analgesia (Brook et al. 2017), antibacterial (Cushnie et al. 2014), antifungal activity (Khan et al. 2017), antifungal activity (Thompson and Nidorf 2018), anticancer activity (Manayi et al. 2019) and antiviral activity (Xu et al. 2019). Among the antiviral alkaloids, the activity of berberine has included chikungunya, human cytomegalovirus (HCMV), and Hepatitis C Virus (HCV), dengue virus tomatidine (DDV) (Hung et al. 2019), human immunodeficiency virus (HIV), michellamine B (HIV) (Luganini et al. 2019) flu oxymatrine against influenza (Varghese et al. 2016) A virus, and zika virus palmitin (ZV) (Diosa et al. 2019). Furthermore, alkaloid-rich plants also have antiviral activity, including Peganum harmala L. seeds, which can block the influenza A virus (McMahon et al. 1995), and root tubers of Stephania cepharantha Hayata, which promote the lifespan of herpes simplex virus type 1 mice (HSV1).
Alkaloids as ACE-2 receptor inhibitors
The synthetic derivative of quinine (CQ) and Hydroxychloroquine (HCQ), an alkaloid extract from Remija and Cinchona (Rubiaceae) species bark used in treating malaria, is hydroxychloroquine and chloroquine. All three chemical species are hazardous to humans, leading to arrhythmias, reduced heart function, and single- and ongoing hypotension (Dai et al. 2018). In adults, it may become poisoned with a single intake of quinine in a range of 10 to 15 g while chloroquine and hydro-chloroquine vary between 2 and 5 g, so highlighting a threat that can be produced if an effective dose assessment is not made properly. For over 3 months, a variety and public exposure of claims concerning the use of hydroxychloroquine and chloroquine were based on provisional drug recurrence data claiming the medicine was “the cure” of COVID-19 (Ho et al. 2019). This arrogant news has impacted many people with no medical advice on the ill effects of self-medication (Moradi et al. 2017). The use of HCQ and CQ whilst fighting with COVID-19 remained in the preliminary test stage, although the global population is anxious to offer a dependable remedy to the scientific community (Moradi et al. 2017; Thanacoody 2016). Such reliable treatment calls for numerous randomized safety and clinical assays with unbiased data acquired from studies of various ethnic backgrounds that are difficult to perform in light of the alleged underreporting of COVID-19 in some countries.
Molecular modelling of a Chinese medicinal plant for the treatment of COVID-19 has been described as SARS-CoV-2 inhibitors by ACE2 extracts and alkaloids of Veratrum nigrum L. 55. Most of the alkaloids suggested are Liliaceans, such as Erioriocapitella hupehensis, pseudo jervine, and imperialize. However, Veratrum species are known to be hazardous when taken after decoction, due to the occurrence of a certain class of alkaloids which are major compounds or minor in their chemical profile. However, human poisoning instances are rare and are usually accidental (Gautret et al. 2020). The analogue of jervine, a poisonous alkaloid, is one of the alkaloids reported as anti-SARS-CoV-2 by molecular docking. So, great attention should be taken when evaluating the usage of Veratrum species notwithstanding the presence of certain putative anti-COVID-19 alkaloids.
It was proven that Lycoris radiata (Amaryllidaceae) extract exhibits strong anti-SARS-CoV antiviral activity in 2005 utilising the MTS assay coupled with virus-induced cytopathic impact. The active molecule of Lycorin with alkaloid structure, potentially shows antiviral characteristics with an EC50 value of 15.7 ± 1.2 nM, is the active ingredient of this extract. These results have shown that lycorine can perhaps be an important development prospect as a new antiviral drug (Tasnim et al. 2020). Another study reports the potential in vitro inhibitory activity of lycorine against coronavirus replication such as HCoV-OC43 (EC50: 0.15 μM), MERS-CoV (EC50: 1.63 μM), and HCoV-NL63 (EC50: 0.47 μM). Additionally, lycorine can diminish the viral load in the central nervous system of BALB/c mice and protect against HCoVOC43-induced lethality.
Pilocarpine from Pilocarpus cearensis Rizzini is the most known member of this class. It is used as a medication in ocular preparations to treat glaucoma. In plants such as Leguminosae, Convolvulaceae, Boraginaceae, Compositae, Poaceae, and Orchidaceae, the pyrrolizidine alkaloid, having the necene base, is present solely. Heliotrin, echinatine, senecionine, and clivorine are some of the most well-known members of this class and are biosynthesized in herbivores. These disorders, such as liver cancer, are hepatotoxic. They are used to treat diabetes and cancer (Funck-Brentano and Salem 2020) because of their glycosidase inhibitory action. Aza is a group of five members of the pyrrolidine alkaloids generated from ornithine and lysine. Some members of this class were Hygrine, the Cusco Hygrine. Biological studies of this class have shown that several of these chemicals have shown important antifungal, antitubercular and antibacterial activity (Fig. 1).
Carapichea ipecacuanha roots (the Rubiaceae family) have the principal active element in alkaloid structure, which has anti-protozoal activity and may provoke emetine. The inhibition of coronaviral replication, such as HCoV-OC43 (EC50: 0.30 μM), MERS-CoV (EC50: 1.34 μM), and HCoV-NL63 (EC50: 1.43 μM), is strong in-vitro. Emetine can also prevent Thionyl chloride MERS-CoV in the host cells (Fig. 2).
Dopamine and secologanin that form N-deacetylisoipecoside (S-form) and N-deacetylipecoside begin the reaction of biosynthesis (R-form). S-form then passes by a reaction of the type Pictet-Spengler and the sequence of O-methylations followed by removal of glucose and the formation of proemetine, using O-methyltransferases and the glycosidase. The final product is then made using a 7"-O-methylation to produce cephaeline and 6"-O-methylation to produce emetine in succession
Tylophorine and several related analogues are obtained from Tylophora indica with phenanthroindolizidine alkaloid structures (Asclepiadaceae). The powerful inhibitory effects of coronavirus replication include Tylophorine (IC50:58 nM) and 7 methoxycryptopleurine (IC50:20 nM.). Tylophorine was also discovered to target viral RNA replication and cellular JAK2, media-dominant NF-ŢB activation at nanomolar concentration, which is a frequent pro-inflammation reaction of host cells to viral CoV infections (Cheng 2020) (Fig. 3).
Initially, started with phenanthryl aldehyde and substituted by phosphonate then the synthetic process is to be followed to obtain phosphonate than the reduction of potassium borohydride and lithium chloride yields allyl alcohol gives chloro alcohol than treated with sodium azide than the formation of formamide takes place which finally gives tylophorine
Alkaloids produced in bisbenzylisoquinolin from the roots of the Stephania tetrandra (Menispermaceae family) comprising anti-cancer, anti-inflammatory drugs and antioxidants, which have various biological activities (Cronemberger et al. 2012). Tetrandrin, IC50: 14.51 μM, fangchinoline (IC50: 12.40 μM) and cepharanthine, with potential antiviral action against the infection of HCoV-OC43 and viral replication suppression, are the primary active S. tetrandra Alkaloids (Li et al. 2005). The cytopathic effects of HCoV-OC43 in humans with EC50 values of 295.6, 919.2 and 729.7 nM, respectively, are inhibited by the alkaloids, tetrandrine, fangchinolines, and cepharanthine. The CC50 values of 15.51, 12.40, and 10.54 μM and SI values > 40, 11, and 13 were detected and demonstrated in the Medical research cell strain (MRC-5) cells the cytotoxic effects.
Homoharringtonine is a cytotoxic alkaloid that was originally identified from Cephalotaxus hainanensis of the Cephalotaxus (Taxaceae family). The FDA has approved the therapy of chronic myeloid leukaemia medication. Homoharringtonin shows considerable antiviral action in many species of lowest IC50 humans and animals (12 nm) (Shen et al. 2019).
Isatin (1H -indole-2,3-dione) contains different pharmaceutical properties such as anti-malaria, anti-allergic, and antimicrobials, and it is an oxidised indole derivate isolating itself from plants such as Strobilanthes cusia, Isatis tinctoria, Couroupita guianensis, and Calanthe (Yang et al. 2010). Isatin derivatives potentially inhibit rhinovirus 3CLPro has been shown in recent studies (Yang et al. 2017). Therefore, it is possible to promise isatin derivatives for innovative COVID-19 treatment in similar protease structure and SARS-CoV 3CLPro inhibitors in low amounts (Weber and Opatz 2019; Kim et al. 2019). Effective alkaloid structures under COVID-19 therapeutics.
Southern African species, Ziziphus mucronata, which has not been investigated for its antiviral activity; however, cyclopeptide alkaloids isolated from Z. jujuba are investigated to produce inhibition of a porcine-related coronavirus (porcine epidemic diarrhoea virus (PEDV)), with SI values ranging from 7.98 to 47.11 on Vero cells (Cao et al. 2015).
The screened performed test Gyebi et al. (2020), series of alkaloids with molecular docking and absorption, distribution, metabolism, excretion, and toxicity (ADMET), a variety of alkaloids and terpenoids produced from Afrikan plants as possible inhibitors of 3CLPro. The results showed that 10-Hydroxyuelbarensine, Cryptoquindoline, 6-Oxoisoiguesterine, and 22-Hydroxyhopan-3-one may be strong drug-like inhibitors of SARS-CoV-2 (Khan and Maalik 2015).
Oxyphoridine is one of the alkaloids isolated from the Chinese medicinal plants Sophora alopecuroides and Siphocampylus verticillatus and belongs to the quinolizidine alkaloid group. This alkaloid has a range of pharmacological activities, particularly in oncology, and is characterised by antioxidant stress, significant anti-inflammatory effects, and anti-apoptosis (Webber et al. 1996). Although researchers have not been very productive in researching the anti-viral capabilities of this alkaloid movement, a new study found inhibition of SARS-CoV-2 oxysophhoridine replication in cell culture (EC50 0.18 μM and CC50 > 40 μM) (Chen et al. 2005b).
Isatis indigotica is a customary Chinese medication utilized in clinical treatment for its target of viral properties in the treatment of infections like flu, hepatitis, and encephalitis (Liu et al. 2014; Kang et al. 2015), just as aggravation. In one investigation, indigo—a significant compound of I. indigotica root extricate—showed intense antiviral movement against Japanese encephalitis infection (JEV) (Gyebi et al. 2020) in vitro study replication in a portion subordinate way. Besides, the season of expansion tests showed that indigo displays a solid antiviral impact previously or during disease, yet not after viral cell passage (Rui et al. 2014). I. indigotica root was also used regularly to prevent and treat SARS in China, Hong Kong, and Taiwan during SARS-CoV epidemics (Yao et al. 2012). Indeed, both the I. indigotica roots extract and the indigo has shown a substantial inhibitory impact on SARS-CoV within the micromolar range (Lin et al. 2005). Similar to other I. indigotica root chemicals, indigo inhibits 3CLpro’s cleavage actions—a viral replication enzyme mediating dose-dependent proteolytic in coronavirus replicase polypeptides (Zhang et al. 2020). In the conclusion, indigo IC50 values for 300 μM and 752 μM free and cell-based testing were reported; this is indicative of the efficient operation of indigo 3CLPro blockers. Finally, a 7.4 mMin Vero cell CC50 suggesting that indigo is not hazardous to Vero cells was reported (Qin et al. 1998). This opposes Chang and his fellow students’ notion about the mode of action for indigo (2012), but can only be based on the investigated virus or the experiment. To clarify the exact mode of action of indigo, further investigations of different viruses and systems would be required (Fig. 4).
Strobilanthes cusia is a conventional medication utilized in India, Thailand and the southern portions of China. In the history of flu, encephalitis B, viral pneumonia, cerebrovascular epidemic meningitis and mumps have been utilized as the root (Chang et al. 2012; Lin et al. 2005). The two main chemicals isolated from S. cusia leaves are tryptinghrin and indigodole B (Berry et al. 2015). The compounds showed high antiviral efficacy in HCoV-NL63-infected cells in the Tsai and colleagues study (2020) in lowering both CPE and virus yield (IC50 values of 1.52 μM and 2.60 μM respectively for tryptanthrin and indigodole B). Further significant trypanthrin viricidal activity (IC50 = 0.06 μM) and indigodole B were reported (IC50 = 2.09 μM). Independently, the authors discovered tryptanthrin as the main antiviral ingredient of the methanol extract from S. cusia leaves with strong activity against HCoV-NL63. Finally, it was revealed that tryptanthrin interferes with in vitro replication in the early and late phases of HCoV-NL63, blocking viral RNA synthesis along with the action of papain-like protease 2 (Shahni and Handique 2013).
Although, customary medications in India and China Tylophora indica and Tylophora ovata are used, natural compounds first obtained from T. indica plants have shown wide in vivo activities against numerous disorders such as inflammation, SARS replication, one of the characteristics of pathogenic coronavirus infection, and are susceptible to inhibiting global protein synthesis (Gu et al. 2015). Interestingly, HCV replication was also inhibited by tylophorines. Previously, results have revealed that Tylophorines are powerful inhibitors of several coronaviruses, such as SARS-CoV, the mice hepatitis virus (MHVs), and transmittable through Gastric routes virus, whether isolated from plants or artificially produced Transmissible gastroenteritis virus (TGEV) (Pham et al. 2012). Clinical data had described the new and strong anti-CoV medicines for the treatments of TGEV and SARS-CoV infections as natural tylophorinine, and Synthetic Tylophorin compounds. Furthermore, the tylophorine compounds demonstrated high efficacy for reproductive anticoronavirus, finally preventing virus-induced apoptosis and consequent cytopathic effect in vitro cells. Yang and colleagues showed that such chemicals have a target of viral RNA to understand their use of tylophorines-based compounds, thereby reducing the TGEV replication (Lee et al. 2012; Lin et al. 2005).
In silico approaches to SARS-CoV-2 of subunit S1 ACE2 receptor interaction with s-protein is blocked after a drug molecule is attached to the s-protein and makes it incapable to infect the target cells
In a prior study, various medications have been discovered to be dynamic against the S-Protein Virus Receptor from a high-performance virtual screening methodology approved by the FDA LOPaC study house (Choudhary et al. 2020). The viral S-protein receptors location was identified as KT185, KT203, GSK1838705A, BMS195614, and RS504393. For example, molecular modelling simulated studies were performed using the S-RBD SARSCOV-2 subunits of amino chains using Libdock protocol in Discovery Studio 2.5 Software to get insight into the binding mechanism and critical molecular interactions of selected Alkaloids.
To forecast biological activities and provide additional insight into binding guidelines and interacting, an investigation of its binding modes has been carried out. S1-RBD alkaloids might interfere with viral fixation to host receptors and thus, restrain infection appearance into the host cell. The investigation was therefore done with the purpose that the interaction and binding affinity of such alkaloids with the S1-RBD of SARS-CoV-2 were identifiable and understood.
Our study on the particular alkaloids and reported SARS-CoV-2S1-RBD compounds is based on the results. The alkaloids chosen were used to grade the libdock ranging from 64.126 to 109.10 kcal/mol in KT185, KT203, GSK1838705A, BMS195614, and RS504393, while the reported earlier libdock scorer was 95. 9.10.43, 7.91.75 and 120 kcal/mol. This finding showed increased binding stability for the complexes of alkaloids-S1-RDB. S1-RDB also preferred the interaction with the majority of alkaloids, in particular homoharringtonine, which showed high docking range. There were three hydrogen bonding acceptors Ser494, Ser494, and Gln 493, and two hydrogen binders with Tyr453 and Pro491 for homoharringtonine interacting with S1-RBD. Hydrophobic interactions involving Leu455, Lys452, and Phe497 were also observed. Also, the high docking rates with cepharanthine can be attributed with Arg454, Arg457, Lys458, Lys458, and Ser469 to the production of cinq hydrogen bonding acceptors and with Gln474 one hydrogen bonding donor. Similar findings have been observed for all dock configurations, such as Leu455, Phe486, Asno487, Gln493, Ser494, and Tyr495 (Nakagawa et al. 2011), and Gly496, in a Binding Region for SRS-COV-2 with the interplay of various amino acid positions. This shows that certain chemicals are very likely to attach to SARS-S-RBD COV-2’s protein that prevents them from attaching to the host cell (Salehi et al. 2019).
Naturally occurring secondary metabolites against HCOV
Terpenes are secondary metabolic naturally occurring association aggregates with various probiotics properties. Terpenes are regular, unpredictable natural plant compounds extricated from natural sources that involve merely hydrogens, oxygen, and carbon molecules, essentially extricated. Terpene is a chemical attractant or chemorepellent in plants (Ero et al. 2003). The distinctive natural and pharmacological effects are used worldwide for the therapy of several diseases. Terpenes result in cytotoxicity, hostility to inflaming, inhibiting growth, and antiviral characters and they likewise help in the therapy of uncontrolled malignant growth and heart-related problems. Terpenes were subdivided into monoterpenes, sesquiterpenes, diterpenes, sesquiterpenes, and triterpenes depending upon their structure in the carbon chain. Perillylalcohol (monoterpene), forskolin (diterpene), and ursolic corrosive (triterpene) have a place with this gathering of dynamic compounds with remarkable and important properties (Zeng et al. 2011).
There is by all accounts a deep connection between cannabis and terpene composition. As a principle, an optional intermediate product of metabolism cannot be utilized as chemotherapeutic markers (Andre et al. 2016). Furthermore, they were characterised as per quantity or numbers of carbon they have popularly called isoprene units. 15 C sesquiterpenes, 10 C monoterpenes, 30 C triterpenes were found in cannabis like the leaves, roots, and blossom among monoterpenes, we can recognize. The mono and terpenes have been found in parts of the plant, like the leaves, fibrous root, and floral part. In between monoterpenes, we can recognize the predominant constituent. The major organic terpene constituents: D-limonene, linalool terpinolene, C10H16 (pinene). The significant amounts of sesquiterpene, including trans-caryophyllene and αlpha-humulene, can be detected in isolated cannabis. Cannabis roots contain triterpenes. They are Epi-friedelano as well as pentacyclic compounds in cannabis fibres these substances. Sativa (Hemp) oil has been recognized for cycloartenols, dammaradienols, and β-amyrin (Richins et al. 2018).
MEP and MVA as biosynthetic pathways
Biosynthetic processes for plant-based terpenes, as the plastic pathway for the synthesis of the monoterpene, diterpenes, and tetraterpenes for methyl methylrithritol phosphates (MEP). The first cytosolic corrosive Melavonic acid (MVA) route is connected to sesquiterpene and triterpene. The first, the cytosolic mevalonic corrosive (MVA) pathway is engaged with the union of sesquiterpene and triterpene. The reaction enhancer substance of the cycle is acetyl coenzyme and pyruvic acid. Later on, converted into (IPP) isopentenyl diphosphates and afterward dimethylallyl diphosphate (DMAPP) formation take place with the help of isomerization. Inside MEP passage, accumulating DMAPP and IPP and arranging the GPP, a monoterpenoids precursor. Two IPP atoms and one DMAPP are combined with a farnesyl diphosphate configuration on the MVA route (Solymosi and Kofalvi 2017) (Figs. 5, 6).
Volatility of terpenes
Some terpenes, for example, limonene and camphene, are depleted specifically from plant parts by their unpredictable nature. Natural circumstances and plant growth affect the measurement and circulation of terpenes produced by the plant (Hazekamp et al. 2016). Terpenes are mainly extracted from cannabis having a huge number of organic characteristics (Elzinga et al. 2015). Nerolidol accounts for 0.09% of Cannabisterpenes, dynamic in opposition to Plasmodium vivax or myriad. The in vitro, immunostimulating, and anxiolytic effects of limonene are cytotoxic. β-amyrin was perceived as pain relief, inflammatory and minor tranquilizers substance. α-Pinene exhibits improvement in the brain cells as tetrahydrocannabinol (THC), which is preventing acetylcholinesterase, has an unfair effect on memory gaps. Linalool, also found in Lavandula, contains Angustifolia, tranquilizers, anticonvulsants, and hostile to inflammatory effects. β-Caryophyllene possesses inflammatory and neuroprotective activities in the copaiba emollient and Piper nigrum for gastric tissue. It also binds to the CB2 receiver. For instance, βamyrin and cycloartenol have Pentacyclic triterpene that demonstrates antibacterial, antifungal, and anti-cancer activity. They may assume a fundamental part in Cannabis natural movement to transform the characteristics of cannabinoids. As already said, the cannabinoids with terpene are therapeutically stronger than themselves due to the prediction of “escort effects” (Grof 2018). Terpene might change the toxicology of THC by expanding the penetrability of the RBC–cerebrum boundary (Rogowska-Szadkowska et al. 2018). In addition, they may likewise influence their capacity to CB1 receptive implementation the pain-relieving and insane impacts of cannabinoids identified with their connection with synapse receptors (Hensel and Zotterman 1951). The impact of cannabis will thus likely be the extent to which cannabinoids, terpenoids, and flavonoids cooperate. No medication preliminaries have been conducted, however, to validate those implications. As of now, compounds of cannabinoids and terpene are engaged with numerous drugs.
Compared to the present natural drug class utilized for human health, tiny molecules represent a rich source of new bioactive chemicals and various chemical scaffolds. Plants provide a significant resource for antibacterial and antiviral medicinal products that have not been used in previously untouched areas and which do not now have a licensed supply of these treatments, but they do so in other medications with comparative abundance. Many medicinal drugs currently on the market have been supplied by compounds, extracted from natural sources, mainly plants and microorganisms. The biodiversity shown in the natural world reflects a richer chemical diversity as well as a large source of new molecules, such as chemical protection or other functions, with biological activity.
Menthol and related p-menthanes from the Lamiaceae
Several para-menthane monoterpenoids of pharmacological significance are found in the Mentha genus (Lamiaceae). Menthol, a significant component of peppermint essential oil “Mentha balsamea wild” recognized to behave (Yin et al. 2018). In comparison with another plant-based organic substance such as capsaicin which has the opposite effect by activating the heat-sensitive TRP vanilloid 1 receptor (Bandell et al. 2006; Voets et al. 2007). Through indirect activation of the opioid receptor, involuntary muscles relaxation, and promotes allosteric sites and activate the Gamma aminobutyric acid (GABA) binding site, the Ca2 + blocking capabilities of 2-isopropyl-5-methylcyclohexanol also give minor discomfort alleviating and soothing results. These pharmacological properties may explain its long-standing and contemporary usage in the treatment of muscle pain and irritable bowel syndrome. It is well known for its use as food flavouring and maintaining oral health products and food preservatives. With some exceptions, ()-menthol production starts with GDP, the direct precursor to the majority of monoterpenes (Caterina et al. 1997). Geranyl diphosphate(GDP) synthase a heterodimer of the first component to be copied from peppermints (Mentha balsamea wild) and (Mentha spicata) generates GDP through IDP and DMAP condensation with large catalysis and a smaller chain length. 1-Methyl-4(prop-1-en-2-yl) cyclohex-1-ene synthases operates on GDP to construct the closed cyclic olefinic monoterpenes. Then, at C3, ()-limonene-3-hydroxylase adds alcohol works to create ()-trans-isopiperitenol. The enzyme ()-trans-isopiperitenol dehydrogenase then oxidises to -trans-isopiperitenone, which is then reduced to ( +)-cis-isopulegone. After that, ( +)-cis-Isopulegone isomerase produces ( +)-pulegone (Galeotti et al. 2002). After that, -menthol is generated via a final reduction by ()-menthone: -menthol reductase. The seven following processes start in plastids, moreover transfer to ER, later on, followed by a powerhouse of the cell, hence ends in the intracellular fluid (Lau et al. 2014). Finally, the secretion takes place in the oil gland’s subcuticular warehouse. Menthol production in mint glandular trichomes is predominantly regulated at the transcriptional level (Simre and Tack 2018) The cytotoxicity of pennyroyal (M. pulegium) is at its peak ( +)-pulegone concentration, it is changed identically to ( +)-menthofuran in the human liver by distinct P450 enzymes. Pennyroyal’s uterine contraction-inducing, hepatotoxic qualities are owing to its bioactivation, which explains why its essential oil has been used as an abortifacient from 421 B.C. regardless it is harmful in potent form. Biotechnological techniques have succeeded in reducing ( +)-menthofuran concentration in mint by silencing ( +)-menthofuran synthase while improving the overall efficiency of the required ()-menthol by modulating the second enzyme of MEP process is DXR (Fig. 7).
Terpenes effective against SARS-CoV-2
Although some of the above-mentioned terpenes were tested against the covid and its enzymes, many lacked inhibitory study in either the SARS-CoV or SARS-CoV-2 viruses for any of the Mpro and PLpro enzymes. Whether the known SARS-CoV protease inhibitors maintain their inhibitory powers against the SARS-CoV-2 proteins is worth researching regardless of the high level of similarities between the two viruses (Turner et al. 1978). The results from the dockage of the 34 terpenes under study are reported in (Mpro) and S2 in additional materials (PLpro). For all 34 chemicals in both the receptors and filtered using a process detailed in the Methods section, molecular docking solutions were found to keep those most likely for each compound. In this process, the visualisation of the projected ligand posing was performed to discard those beyond the binding cavity and set of very similar compounds. The possible Terpene–Mpro and Terpene–PLpro complexes were therefore 65 and 49 respectively. More complexes are found from PLpro due to its larger binding capacity (Fig. 8).
Monoterpenes: cannabinoids as a therapeutic agent
The term ‘cannabinoids’ initially refers to a class of prenylated phenolics chemicals originating from Cannabis spp. Yet today it refers to any ligand susceptible of specifically interacting to human cannabinoid receptors, including breakdown product cannabinoids with little identical structures plant extracted, terpenophenolic belonging (Degenhardt et al. 2017). This section focuses solely on cannabinoids derived from plants. In Glandular Trichomes, however, cannabinoids, such as p-menthane monoterpenoids accumulating in the Lamiaceae are mostly found within the calyxes and bracts of flower buds in Cannabis spp. Cannabinoids have a monoterpene-based C10 GDP backbone, a resorcinol ring of polyketides, and a variable-length, usually 3- or 5-carbon acyl chain (Fellermeier and zenk 1998) C. sativa's two most well-known cannabinoids are 9- (THC) and cannabidiol (CBD) are two compounds recognized for their psychotropic and pain-relieving qualities, accordingly. They have many early metabolic stages before diverging at the core intermediary cannabigerolic acid (CBGA), the main stage in cannabis synthesis. GOT generates CBGA via prenylations of (OA) (Taura et al. 2009). Olevetolic acid is the outcome of the fusion of hexanoyl-CoA with three malonyl-CoA isomers, which needs the simultaneous operation of olivetols synthesis in addition to olivetolic acid synthase (Gagne et al. 2012). The genes involved in the production of hexanoyl-CoA are yet to be identified. CBGA can subsequently be oxidatively cyclized to CBD acid or Tertahydrocannabinolic acid (THCA) by the activity from the first and second two important biomolecules. Primarily, CBDA hydroxylase, a flavin-containing biomolecule, repeatedly oxidises the glycosyl groups via a method requiring deprotonation of geranyl chain’s final methyl group, leaving a free propylene group. CBGA, on the other hand, may be subjected to a comparable oxidative cyclization by THCA synthase (Taura et al. 2007). Here, the C10 hydroxyl group of the alkylresorcinol chains strikes the propylene back end and forms the third ring. In this situation, the carbocationic intermediate deprotonation occurs. THCA is distinct from CBDA by this extra ring while the mechanics of the enzyme are essentially the same. The physiologically active components CBDA and THCA are subsequently subjected to spontaneous decarboxylation (Sirikantaramas et al. 2004). The pentyl-side chain of the resorcinolic acid ring is characterised by CBDA and THCA. But it is also known as additional pharmacologically active cannabinoids with various length acyl groups. CBDA and the THCA propyl substituted analogues are available in the form of CBDVA and tetrahydro canabivarinic acid CBDVA. It is believed that they are composed of the same enzyme, which yields its second- and third-cyclical pentyl equivalents (CBDAS and THCAS, accordingly). The propylcannabinoid thereseolic acid components are divarinolic acid instead of OA (Taura et al. 1995), and the alkyl group is supposed to be made of butanoyl-coA. The discovery of which was a consequence of the plant affinity to CB1. This G-protein combined receptor acts in an inhibition fashion to limit the pre-synaptic neuronal calcium influx necessary for the release into the synaptic cleft of dopaminergic neurons such as glutamate. CBD’s objectives are less defined, however, they have both proven their efficacy in epilepsy treatment (Meijer and Hammond 2020). Cannabis spp. is not restricted to cannabinoids sensu stricto, pharmacologically active terpenophenolics. The consequence of a transfer to a methyl-flavone chrysoeriol ring from either a geranyl or dimethylallyl group are two prenylated flavones, cannflavins A and B (Gulland and Mem 1952) The alcohol groups’ location on the aromatic ring is the same as a resorcinol benzene cyclic compound of OA during the production of CBGA concerning the site of prenylation. Lease. The two cDNAs that are involved in the synthesis of cannflavins have recently been identified, namely O-methyltransferase, which transforms luteolin into chrysoberyl, and aromatic prenyl transferencias that accepts either GDP or DMADP as prenyl Donor, respectively. Its analgesic and anti-inflammatory action stems from two enzymes inhibited in the inflammatory trajectory of E2 prostaglandin. The recent identification of the cannflavin pathway’s cDNAs will allow a closer examination of the therapeutic capacity of bearing pain alleviation and chronic sensitivity management.
Sesquiterpenes Artemisinin
Wormwood has shown particularly effective for the treatment of malaria, as the source of sesquiterpene endoperoxide artemisinin. Artemisinin has an unequalled ring of endoperoxide and is a sesquiterpene lactone with anti-malaria characteristics. There is currently no obvious method for acting and numerous options were suggested (Bulduk et al. 2015). In most cases, endoperoxide degradation is involved in a heme-dependent process in which carbon centred radicals are formed, and numerous goals such as heme and proteins are then alkylated. The participation of heme complies with the artemisinin specification for Plasmodium spp. The direct source of parasite toxicity is unknown, however, interference may be associated with hemocyte conversion, which is controversial. This is a theory (and reviewed in reference Fossati et al. 2015). At the biochemistry and molecular levels, the early phases of artemisinin production in gland trichomes were understood, however, the exact mechanism of subsequent phases remains unknown. Current efforts are currently focused on regulating this route to enhance the direct synthesis of artemisinins in herbs. The path starts with converting the Farnesyl diphosphate (FDP) with amorpha-4,11-diene (Flores-Sanchez and Verpoorte 2008). The existence of this olefin and its derivations oxygenated by artemisinic alcohol and aldehyde, dihydroartemisinic, and aldehyde, as well as its biochemical characteristics and the metabolite profiling of plant tissue recently identified a reductase Double bond reductase (Dbr2) artemisinic aldehyde, which prefers a reduction to dihydro-artemisinic aldehyde (Shoyama and Hirano 1984). Aldehyde dehydrogenase 1 (Aldh1) then converts this substratum to dihydroartemisinic acid. The rest of the stages include the creation of the photo oxidant ring of dihydroartemisinic acid. However, there are presently uncertain specified mechanisms as possible participation of other molecular characteristics (Fig. 9).
Sesquiterpenes Thapsigargin
Thapsigargin is an extremely important 6,12-guaianolide sesquiterpenes lactone derived from the West Mediterranean Bay, Thapsia garganica (Apiaceae). Contact dermatitis is produced by its resin. It’s often known as “deadly carrot.” It has been widely employed for rheumatic, impotence, and colds for Native North Africans medicinal herbs cultures and is used in Algeria for cardiovascular causes dating back to the nineteenth century. Histamine releasing abilities have been documented by the late 1970s, and their protein target was subsequently identified as the Ca2 + /ATPase pump Sarcoendoplasmic reticulum calcium transport ATPase (SERCA) sarcoplasmic reticulum. During the muscular contractions/relaxation cycle, SERCA is important for Ca2 + flowing into the sarcoplasmic reticulate in myocytes. Thapsigargin through SERCA restriction thereby prevents the release of Ca2 + ions from the cytosol by limiting muscular relief. Thapsigargin typically leads to endoplasmic reticular stress and as a consequence, it leads to apoptosis during autophagy to autophagic fusing well with a lysosome. Therefore, its capacity to altered Ca2 + homeostasitics in human cells has indicated a possible function in the treatment of cancers but it has until recently prevented further development as an anti-cancer medicinal product due to its high cytotoxicity and lack of tumour cell specificity. Thapsigargin was successfully combined with a prostate-specific antigen (PSA) to productively generate prostate cancer-targeted medicine. Denmeade et al. In Phase II clinical trials of hepato-cellular carcinoma (Matsuda et al. 1990), Mipsigargin, Guaianolide dependent version of PSA coupled strategy focusing on rigid tumours. Thapsigarganic yield is low (< 0.5% by weight) at T. garganica and the plant is hard to culture, which makes the details of the genes and enzymes involved instrumentalize production on an industrial scale. Our present understanding of Thapsigargin biosynthesis finite, and absolute DNA cloning and biochemical activities have only been achieved for the first time. The TgT PS2 sesquisterpenes synthase transforms sFDP into sesquiterpene alcohol in the committed step of the pathway (Herkenham et al. 1990). Client C6’s pre-existence permits the lactine ring to develop in line after triple oxidation, which is the biosynthesis of costunolide, requiring the development of two independent cytochrome P450s for alcohol and acid groups before factorization. An Oxydase Transcripts the cells present on the surface of the body gut secretion canal of T. garganica root sections have recently been identified for TgTPS2 and TgCYP76AE2. These discoveries were supported by the imaging of mass spectrometry in Thapsigargin structures resembling secretory ducts, which heavily involved epithelial cells in Thapsigargin biosynthesis. Further reactions to the already elucidated level of thapsigargin includes, 10-strong epi-dihydro costunolide ring closure in 5 and 7 rings, six further hydroxylases and four alkanoylation contradictions with detection in root extract more mediators that may give hints about the prevailing in vivo reaction sequence could be an indicator of a structure of metabolons that efficiently transfers metabolic intermediates across active sites (Gutzeit and Ludwig-Müller 2008). Improved methods to bioinformatics and transitory expressive technologies that minimise the need to produce synthetic substrates will certainly play a part in clarifying the rest of the phases.
Paclitaxel as medicinal diterpenoid
Taxol is the designated preference of Paclitaxel; it was newly and primarily extracted from the pacific yew tree and inherently characterised. The anti-neoplasmic substance was used for a particular purpose. Because of the complexity of its functional groups, considerable efforts were needed to clarify its structure. Paclitaxel comprises 8 functional oxygen groups, including 2 with acetylations, and oxetane ring, an attachment to the definite site on parent ring on Carbon 13 consisting of hydroxylation and benzoylation-modified β-phenylalanyl group. The evidence for their mechanism of action was given a few years later as a mitotic stabiliser and inhibitor but Food and drug administration (FDA) approval was only issued in 1992 and 1994 respectively for the use of refractory and metastatic breast cancer. The extraordinarily low amount of paclitaxel in tissue has caused a considerable delay, limiting the stock of what is currently the most marketable anti-carcinogenic agents with yearly combined sales amounting to almost 10 trillion USD (Kaplan and Springs 2008). The order of various hydroxylations and acetylations remains still unknown. Feeding studies reveal some paclitaxel routes operating within the plant together with several substantial wrongs end in less biological taxoids, such as ( +)taxus in, a rich non-bioactive taxoid that provides a generic substratum that will easily be available to test the cytochromes P450s activity (Friedman and Devinsky 2015). There may be some varying orders of reactions on the natural route. Below, we underline what the major road to paclitaxel is usually considered. GGDP is usually regarded as the precursor for all diterpenoids. The GGDP synthase activity combines one DMADP unit with three IDP units from the MEP trajectory. A cycle of GGDP to taxa-4(5), 11(12)-dioene is the primary committed reaction for paclitaxel production. This olefin subsequently serves as a substratum for taxadiene-5αhydroxylase, an ER-localized P450 cytochrome using radical devices, abstracting an H atom from the methyl group of C20, forming a radical allylic to which the oxygen adds to generate taxa is the result of acetylation of such alcohol in the cytoplasm by taxadene-5α-ol-O-acetyltransferase. Strange uncertainty as to the order of eight future steps results in numerous plausible intermediates converging towards a hypothesis that now includes eight functional oxygen groups. Ash has the four-member oxetane ring here, this precursor now offers binding energy with β-Tubulin; hence, it is crucial for paclitaxel’s pharmacological action. Oxetane ring formation studies have offered two mechanical reasons for its production: firstly, a 5 acetoxy-4(20)-epoxy which is then rearranged into a ring oxetane (20),11(12)-dien-5α-yl-acetate. The other indicates that 10-deactylbaccatin III 10β-O-acetyltransferase (DBAT) downstream acetyltransferase might be acetylated the tertiary C4 location independently on oxethane (Barrett et al. 1986; Rea et al. 2019).
Movement of the NT-VRL-1 unique to HCoV-229E
The current study aimed at evaluating the antiviral movement of the NT-VRL-1 unique terpene definition to HCoV-229E and the technique of antiviral activity of the HCoV-229E while on the outside the expansion of cannabidiol (CBD). NTvRL1 consists of thirty conventional cannabis terpenes as well as other plants, where beta-caryophyllene, eucalyptol and citral are the main ingredients (Formukong et al. 1998). The restorative action of these mixtures was assessed as far as the cytopathic impact saw under an upset magnifying instrument and artificial insemination feasibility XTT test.
Cytotoxicity of the molecule -MRC-5
Atoms in 96-well plates in the lowest basic medium Eagle have been plated with 1 to 104 cell/well plated, augmented with 10% serum foetal calf, and have subsequently hatched with 37 to 3% CO2 (Werz et al. 2014) at the same time. The medium was removed the following day with 100 μL of EMEM added to the cells, and the mixes were augmented by 1% foetal calf serum. For each expected treatment, the corresponding emphasis was tested. CBD: 2 μg/mL, 0.05 kg/m3, 0.01 kg/m3. NTVRL1, five microns/ml, ten microns/ml, fifty mg/ml and 100 ml, 500 micronizing and 1000 μg/ml of glycyrrhizin: For another 72 ± 2 h at 34 BC 5% carbon dioxide cells are hatched. The cells were finally under XTT screening. In light of the aftermath of this study, the following mixes were determined: NVRL (0.02 kg/m3 0.05 kg/m3 and 0.01 kg/m3), NT-VRL (0.01 kg/m3), NTVRL (0.02 kg/m3) and CBD (0.01 kg/m3), and glycyrrhizin (400 μg/mL) for the efficacy of assessments.
Accuracy of the compound-cells pre-therapeutics
The medium was discarded for 1 day and a hundred μL of an EMEM was supplied to the cells, augmented using mixes already identified as nontoxic at fixations and enhanced with 1% foetal calf serum. The cells were blown to 93.2 F for 60% and 5% CO2 for 50%. Then, 0.001 kg/m3 of median as a choice infection at multiple extents of a grouping of the ineffective portion (1:340 weakening) were included in the cells. Similarly, the process is repeated in a cyclic pattern, cells are hatched for an extra 72 ± 2 has 93 °F and 5% CO2. Under an altered magnifying lens, a microscopic view of cells is determined in every medication at twenty-four, forty-eight, and 72 h post-contamination. An infection-initiated cytopathic impact was seen in examination with the control and cell control. At last, cells were exposed to an XTT assay (Guimarães et al. 2019).
Accuracy of the compound—virus pre-therapeutics
The forthcoming daylight, 0.012 kgm3 of EMEM was extended to the limit. Results increased in 1% of calf serum. The mixtures at focus before were classified as non-poisonous. Hereafter, infections were blended with mixtures of U-formed platter and afterward brooded for 1 h at 93.4°F. At that point, 0.0012 kg/m3 µL medium or infection at multiple extents convergence of ineffective portion was included in the method. Then, 100 µL of the infection + intensified blend was added to the cells after the medium was taken out and tissues were hatched at 33 °C and 5% carbon dioxide considering extra 72 ± 2 h. The infection initiated cytopathic impact was seen in examination with the equal infection cellule regulation. At long last, cells go on examination for the XXT test (Guimarães et al. 2019).
Viability test by XXT assay
To the end of each brooding time, media have been drained from every well, and 1 kg/m3of the new growth phase and 0.05 kg/m3 of XTT reagent have been provided to cells. The ocular thickness (OD) was estimated at 4.5e-7 m (moreover, the shapeless ocular thickness was determined to be 6.2e-7).
The non-cytotoxic convergences of the different mixtures were resolved as the focus that didn’t prompt overabundance cell death, as contrasted with untreated cells compounds preceding immunization with HCoV29E. The practicality of cells that were contaminated with HCoV-29E, in any event, the rationality of the uninfected control cells was dropped to ~ 40%. Cells pre-brood with the complete combination before the infection immunization have protected and enlarged cells (Astani et al. 2010). It is of remarkable interest to enhance new, successful antiviral treatments with little toxicity and few results. The options show significant antiviral potential, low harm, for use as an antiviral specialist, in-plant metabolites like terpenes and cannabinoids (Astani and Schnitzler 2014).
This survey was intended to evaluate the popular counter-movement of terpene NT-VRL1 describing HCoV-229E humans in vitro with or without CBD (Gulland and Mem 1952). This reports on the antiviral action of NT-VRL-1 and shows that the action was improved in conjunction with CBD to propose either a synergetic or an additional effect among terpenes and CBD (Bulduk et al. 2015). This study shows that the action has been improved. Some research has shown that the phytochemicals of cannabis can help inflame experts as possible opponents. Such measures can be especially effective for the management of autoimmune disease and intense COVID-19 breathing issues. The primary examination to explore marijuana plant materials for the use of Covid (Bulduk et al. 2015; Fossati et al. 2015).
Pyrazofurin, certain controls used were a specific antiviral drug that was shown to successfully control the Covids connected to SARS (Page and Maddison 2008). As a result of the positive control, the glycyrrhizin, which appears to have an antiviral effect on the covid virus, is filled out. A method of NT-antiviral VRL1’s activity was checked by expanding the mixture to uninfected lung cells before and after HCoV229E had been vaccinated.
The hour-of-expansion test can help us decide where HCoV-29E replication is restricted by our chemical. Maehle A H 2009 outcomes exhibit anything NT-VRL-1 antiviral impact be generally articulated in pretrials framework, way out show such as mixtures’ antiviral impact depends onwards on anticipation of virus connection and additionally section. Past investigations including glucoside had introduced a medicinal drug component similarly incorporating impedance with capsid encompass designs or covering of viral constructions, in this manner hindering the assimilation of the infection into the cell (Wang et al. 2019). CBD, NT-VRL-1, and positive controls (0.005 kg/m3 pyrazofurine with 0.4 kg/m3 glycyrrhizin) showed a discernible antiviral influence in both premedicate procedures. We also noted a synergistic anti-viral impact which was much more robust than that shown for the positive controls though cannabidiols CBD (0.001 kg/m3) and NT-VRL (0.01 kg/m3) be used simultaneously. Antiviral action against SARS-CoV has seemed to take place at Terpenes. However, terpenes have been introduced simultaneously to the infection throughout earlier exams, and no seasonal precautions have been taken, supposedly, therefore the primary assessment on antiviral activities in terpene and marijuana in opposition to Covid. NT-VRL displayed an antiviral impact, which ought to ideally be fixed up with tissue before infection openness (Guerrero-García and Rubio-Guerra 2018). Alveoli are primary sites influenced by COVID protection medication straight forwardly to alveoli, perhaps utilising inward breath, would be the ideal organization course for this possible remedial arrangement (Cinatl et al. 2003).
Common naturally occurring tannins
Tannins for a long time have been used around the globe for a multitude of health issues. The intake of tannin-rich foods has been encouraged especially in beverages and in the form of various extracts. The existence of tannins is widely distributed in almost all the parts of plants such as seeds, stems, leaves, roots, and fruits. The significance of tannins in the first place lies in their anti-inflammatory activity. The rich astringent processes which are contributed by the high affinity of tannins towards the proteins makes them potent anti-inflammatory properties due to which they aid in conditions like throat infections, alimentary canal disorders such as inflammatory bowel disease, gastritis, enteritis, mouth ulcers. Other than this, they are also helpful in cases of minor burns and heal injured tissues (Cheng et al. 2012). Tannins exhibit the “Tanning effect” which generates a protective film on the surface of damaged tissues. Tannins tend to form hydrogen bonds between their phenolic group and peptide bonds of proteins which ultimately lead to the precipitation of proteins (Ricardo-da-Silva et al. 2008).
Moreover, tannins inhibit certain enzymes effectively like tannins isolated from wood fruticosa exhibits activity similar to topoisomerase II and oenothein B isolated from species of Epilobium inhibits the DNA polymerase enzyme of Epstein-Barr virus (EBV) making it a remarkable antiviral agent. Similarly, it also has distinct anti-cancer activity as it inhibits enzymes like 5α-reductase and aromatase which have a distinguished role in causing benign prostate hyperplasia. Various studies suggest a positive role of tannins in skin, lungs, liver, oesophageal, breast, cervical, prostate cancers as well with quite a few promising mechanisms. Ellagitannins reportedly bind to the protein present on the cells that causes cell proliferation and also induce inhibition of factors accountable for causing metastasis. Moreover, they link with the carcinogens that make carcinogens less potential to cause any kind of mutation (Sepúlveda et al. 1999). Reports also suggest that certain ellagitannins and nobotannins have the property to inhibit enzymes such as poly (ADP-ribose) glycohydrolase which possibly is involved in regulating functions such as replication of DNA, cell differentiation, and gene expression. Also, chebulagic acid present in Terminalia chebula has been reported to possess the property of inhibiting maltase which is an important factor in the development of type-2 diabetes (Yoshida et al. 2011).
In vascular biology as well tannins have distinct roles. For instance, pre-clinical studies show that a tannin-rich fruit named Terminalia bellerica has a remarkable positive effect in the regulation of arterial blood pressure via antagonising calcium pathway. Hydrolysable tannins also possess the anti-hypertensive activity and known to be involved in inhibiting Angiotensin-Converting Enzyme I (Liu et al. 2020). Specific microbial enzymes are peroxidised, laccase, pectinase, glycosyltransferase, xylanase and there are certain kinds of tannins in nature which are known to inhibit these enzymes thus imparting potential anti-microbial effect as well. Along with this, the reported mechanisms include binding with the proteins present on the membrane of microbes and binding with the indispensable metal ions. Tannins also reveal their property of immunomodulation via different mechanisms of action (Ushio et al. 2012). Considering the anti-viral activity, tannins have elucidated promising testimony as potent anti-viral agents.
Tannins considerably have been reported to target potential stages of virus replication in the host such as integration and penetration of virus inside the host cell, assembly of virus components, transportation of essential proteins, polysaccharides, and enzymes, linking with the capsid protein. The tannins are lethal against both enveloped and un-enveloped viruses (Uozaki et al. 1998). Certain tannins especially pseudo tannins have been evaluated against about 16 infections caused by human papillomavirus or HPV where the attachment of HPV is usually inhibited by tannins and also against influenza A virus (IAV). A tannin-rich plant Euphorbia hirta has demonstrated antiretroviral activity in certain in vitro studies (Gyuris et al. 2001). The hydrolysable tannins such as punicalagin, chebulagic acid, strictinin are effective against the Hepatitis C virus and block its entry inside the cell as well as targets the replication of duck, human and porcine viruses (Lin et al. 2002). Most importantly, certain classes of tannins like ellagitannins obtained from the plant Tuberaria lignosa have defined effects against the replication of human immunodeficiency virus (HIV) by inhibiting their entry inside the MT-2 cells in the conducted in vitro studies also according to the current investigations ellagitannins might inhibit the activity of reverse transcriptase. In addition to HIV, ellagitannins also have a pronounced anti-herpes virus effect (Yang et al. 2004).
Not only ellegitannins but certain hydrolysable tannins contained in Terminalia arjuna Linn like casuarinin also produces anti-HSV-2 effect by inhibiting its entry inside the cell (Cheng et al. 2009). The trend of inactivation of entry of the virus inside the cell is also demonstrated by the two already stated hydrolysable tannins named chebulagic acid and punicalagin present in Terminalia chebula Retz, which is also effective against the virus’s glycoproteins (Lin 1997). Usually, the exacerbation of viral infections is also contributed by oxidative stress or the over-production of free oxygen radicals which disrupt the cell’s oxidative homeostasis and hence lead to cell injury. This holds good for specific infections which are blood-based and caused by human immunodeficiency virus (HIV), herpes simplex virus, hepatitis virus, Epstein-Barr virus, and various others. Certain kinds of DNA viruses are linked to the exaggerated oxidative stress causing DNA damage and play an important role in the progression and development of neoplasia. Various studies suggest the potential of tannins against a broad spectrum of viruses. The tannins obtained from the extract of apple polyphenol are useful against the influenza virus—it reduces lipid peroxidation and elevates the oxygen radical absorbance capacity in splenocytes (He et al. 2005). Similarly, tannins found in the extract of Chaenomeles speciosa possess potent anti-viral and anti-inflammatory properties and help remarkably in the management of Avian influenza. So this can be concluded that tannins are not only beneficial in anti-viral therapy but also assist in the combination mechanisms by attenuating the oxidative stress.
Tannins: a possible therapeutic key for COVID-19
Tannins as discussed above are phenolic compounds that are potentially active as antioxidants and antiviral agents. There are numerous studies carried out recently that specifically investigates the role of tannins as potent inhibitors of SARS-CoV-2. Most of the studies are investigated via in silico approaches and the most potent targets were reported to be the main protease of SARS as well as the cellular proteases of the host like TMPRSS2 which integrates the spike protein of this pathogen.
Tannins as inhibitors of COVID-19 main protease MPro
X-ray crystallography has reported that the main protease of SARS-CoV-2 main protease MPro contains 306 amino acids and its active site is composed of an active dyad Cys-His. And the molecular docking which emphasizes the binding interaction between the target’s receptor and ligand reports that certain tannins possess the excellent inhibitory activity of this protease. Certain tools like AutoDock/Vina established the inhibitory activity of tannins like Riboxin, Terchebin, Casuarictin, Punicalin. Even some tannins like Punicalagin possess the binding affinity between (− 9.0 to 0.25) and other tannins as well which is even better than the present therapeutic drugs used for management of COVID-19 such as dexamethasone and remdesivir (Fig. 10).
Derivatives of tannins
Nineteen LigX tannins have been revealed by an interactive affinity study. To implement new allosteric chemicals, it was shown that 19 tannins interact with different tannins simulated selection was completed, as against of COVID-19 Allosteric mandate has been portrayed as a productive strategy to vanquish hopeless hindrance. Spatial arrangement and dock score of the current four top-ranking leaders show that functional residues have a real interaction with the strongest binding affinity Tannins have been found to interact differently with various SARS-CoV-2-3CLpro residues (Chang et al. 1995). The finest hydrolysable tannins in detail include pedunculate that can bind to 12 SARS-CoV-2-3CLpro amino acid residues and have five H Bonding Forces. Above all, pädunculagin engaged directly with the catalytical dyad residues of 3CLpro utilising H-binding forces.
The ability to engage is demonstrated by its ability. Castalin and tercatain also connect directly through arena-arena interactions and H-bonding forces with His41 and Cys145.A, grandinine, granatin, bicornin, and repandusinic acid A A, Roxbin B, and Terchebin interacted with His41 specifically through interacting with H-bonding or arene-arene, and demonstrated their impact on 3CLpro catalytic dyad residues. Results also demonstrated the secondary interaction between certain hydrolysable tannins, such as tellimagradin I, strictinin, punicalin, chebulagic acid, casuarictin, ß-pedunculagin, potentitillin and isoterchesbin, with both His41 and Cys145, which have a partial influence on the 3CLpro catalytic dyad residue A, roxbin B, and terchebin interacted accurately with His41 through interactions with H-subjects or arena, and demonstrated their influence over the 3CLpro catalytic dyad residue (Silva et al. 2016). Results show that there are also some hydrolysable tannins, including tellimagrandin I, strictinine, punicalin, chebulagic acid, casuarictin and ß-pedunculagin, potentitillin and isoterchebin, interacting with both His41 and Cys145 secondary, demonstrating a partial influence of both the 3CLpro catalytic dyad residues.
The vital catalytic residues of the compact spatial site of pedunculagin are stabilised within the pocket with the deepest S-score (− 18.58) and binding with C145, H41, N142, S46, T45, T24, T25, Q189, E166, H164, M165, and M43. Castalin interacted with C145, H41, L27, M49, G143, Q189, N142 S46, T45, T25, and C44. The difference in the binding ability of each tannin with SARS-CoV-2-3CLpro may be due to their structure-based relationship activity. The catalytic residue of the pedunculagin compact site are stabilised with a deep S-score (− 18.58) inside the pocket and are bound with C145, H41, N142, S46, T45, T24, T25, Q189, E166, H164, M 165 and M43. Interactions between C145, H41, L27, M49, G143, Q189, N142 S46, T45, T25, and C44 were carried out by Castalin. The alternative is the ability to bind each tannin can be attributable to the structural relation activity of SARS-CoV-2-3CLpro. At least three novels of safe (Zuo et al. 2005; Serrano et al. 2009), drug-proof tannins were found that are intended to interact with the SARS-CoV-CLpro receptor binding point and catalytic dyads (Cys-145 and His-41).
Pedunculagin, which has a strong influence on the catalytic residue of SARS-CoV-2–3 CLpro (Cys-145 and His-41), has a sense binding affinity with docking score and ADME Tannins among those hydrolyzable tannins (Jo et al. 2019). Pedunculagin is a good soluble substance and is highly absorbed intestinally and has no mutation. This chemical is not a HERG l and HERG inhibitor, therefore cardiac muscle is not damaging (heart muscles). Pedunculagin, tercatain and castalina, from which pomegrans, walnut, Indian gooseberry and oak wood, Melaleuca Quinquenervia leaves, Terminalia catappa and Combretum glutinosum leaves had previously been isolated, have antiviral activities and other biological activities. Rather, it was observed that hydrolysable tannins are degraded by successive acts of several bacterial enzymes to gallic acid, pyrogallol, and Phloroglucinol (Khaerunnisa et al. 2020).
The monomer kinds of hydrolyzed tannins are also gallic, hexahydroxydiphenic acid and ellagic isoterchebin acids. Ellagitannins were reported to release ellagic acid into the intestines of rats after hydrolysis. Caco-2 cells are fed gallic acid with a maximum absorption rate of around 1.27 h in humans via the paracellular route. As for absorption of acid, ellagic acid was discovered from 0.5 to 3 o’clock in humans plasma following oral pomegranate syrup in several writers. The ellagic acid was conjugated after absorption; the conjugated forms were identified in plasma and discharged into the urine with methyl, glucuronyl and sulphate groups (Zhu et al. 2018; Yang et al. 2003) Bacteria, fungus, and yeast can quickly break down gallotannins. In galloyls residues of galloyls esters as well as hexahydroydi-phenoyl, tannases generated by a group of microorganisms are active. Colonic bacteria can metabolise hydrolysable tannins as opposed to proanthocyanidins. The existence of lactobacillus with the different activity of tannase means that during colonic fermentation, gallic acid may be found from gallotannins (Smeriglio et al. 2020).
Ligand characteristics were examined with the LigX MOE tool to certify the drug capability of selected tannins (Qamar et al. 2020). All tannins selected reveal constructive findings and comply with Lipinski’s five standards. The rule sets forth that the permissible components of medications should have about 5 H-bond donors, maximum of 10 H-bond acceptors, and a coefficient of the octanol–water table log P > 5. These results suggest that natural components, including pedunculagin, tercatain, and castalin, found in our investigation may show more valuable adversaries for COVID-19 drug rehabilitation (Kim et al. 2020).
Pedunculagin was simulated in MD and RMSD, gyrator radius (RoG), and the H-bond characteristics were expressed to further evaluate the molecular docking results. There were no evident oscillations in Pedunculagin-SARS-CoV-2-3CLprocomplex regarding the strength of tannin-protein complexes, the usual value of which is RMSD is 1.4 ± 0.01 Å. Normal behaviour, where dense and stable for simulations of 80 NS, has also been proposed for pedunculagin-SARS-CoV-2-3CLpro complex Also, H-bonds, which constitute the main protein stabilisation forces, proposed to remain constant over the entire simulations with no apparent variations in pedunculagin -SARS-CoV -2-3CLpro complex. This study shows that pedunculagin, tercatane, castaline and their sources as well as pomegranates and nuts can be used to help prevent SARS (Chen et al. 2011) as a possible natural inhibitor -CoV-2. Pedunculagin is a hydrolyzable tannin. In medicinal plants, secondary metabolite components are frequently identified. Thus, we projected the pedunculagin pharmacopoeia based on its active groups. Amino-acid residue in the energy point of SARS will also play hydroxy groups (− OH), keto group (= O), and phenolic rings in pedunculagin -CoV-2-3CLpro (Fig. 11).
Hydrolysable tannins are a good approach to inhibit SARS-COV
This investigation has been targeted to identify a strong COVID-19 inhibitor in 19 hydrolyzable structural tannins that could target the major SARS-CoV-2 protease with silicon methods (molecular docking and drug-likeness scan). The conclusions of our study will help researchers to assess the status of COVID-19 in natural therapies (Pillaiyar et al. 2020). Bioactive compounds have gained considerable attention, especially hydrolyzable tannins, which can be used to generate non-secondary medicines without any harm (Liu et al. 2016).
Hydrolysable tannins are also tested to prevent the opposite effects of several chemotherapeutic products as well as to improve permanence and achieve a positive overall health through cancer and can be used as a replacement for cancer supply of medicines (Buzzini et al. 2008). Antiviral effects on B viruses were exhibited by hydrolysable tannins including punicalagine, punicalin, and geraniine by preventing the development of cccDNA which can help to increase the number of innovative drugs to cure HBV infected patients. Similarly, anti-herpesvirus activities by targeting viral glycoprotein-glycosaminoglycans, which can block access and cell-to-cell communication have also been observed, including tannins such as A and B epiacutissimins, castalin, Vescalin, chebulagic acid and punicalagin. Essentially, tannins were suspected of being structurally dependent on the biological activities, including antiviral properties. For instance, the key factors impacting biological activity include additional processes including hydroxylation, methylation, glycosylation, galloylation, and polymerisation (Spelman et al. 2006).
A strong inhibitor against the COVID-19 from 19 distinct structural hydrolysable tannins, which could use silicon methods to target the principal protease SARS-CoV-2 (molecular docking and drug-likeness scan). The results of our investigation will enable the scientists to assess the status of COVID-19 in natural therapies (Pleschka et al. 2009).
Ligand database
The structures of 19 hydrolysable tannins including, castalin (CID99973), bicornin (CID-71308161), grandinin (CID-492392), tercatain (CID-14411424), granatin A (CID-131752596), tellimagradin I (PubChemCID-73179), geraniin (CID-3001497), casuarinin (CID-13834145), strictinin (CID-73330), pedunculagin (CID-442688), punicalin (CID5388496), chebulagic acid (CID-250397), casuarictin (CID-73644), ß-pedunculagin (CID-5320441), potentillin (CID-5315734), isoterchebin (CID-442685), roxbinB(CID-176131), repandusinic acid A (CID147900), and terchebin (CID-3084341) were taken from Pubchem. With independent loads and energy minimization through Protonate-3D and MMFF94X strength chain, the tannin structure was adjusted for docking. In a catalogue of ligands, the adjusted ligands of the 19 tannins were finally used to file docking investigations.
Refinement of 3CLpro of 2019-nCoV structure
The 3CLpro 3CLpro SARS-CoV-2 (PDB:6y84) structure was also recovered with a 2.16 Å resolution from the Protein-Data-Bank. To improve the 3CLpro structure, MOE-09 separated previously associated ligands and H2O substances from the 3CLpro structure, 3D protonation and energy minimization (Kuo et al. 2006). The reduced structure was used in the next steps for further docking.
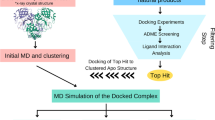
Molecular docking and drug-likeness analyses
The MOE docking tool was utilized to connect 19 distinct structural tannins to the allosteric SARS-CoV-2-3CLpro interaction site (Zhang et al. 2017). The feasible compact binding was found by the MOE-09 locator tool and then used to dock. A scoring job London dG was designed for the ten best-docked positions. Improving the docking process by operating a forcefield algorithm that saves the receptor company has been concluded. Of these, the finest related ligands and components were chosen based on root-mean-square deviation (RMSD), which is frequently calculated in angstrom (Å). The interaction investigation with the Ligand receptor was carried out utilizing MOE-09 LigX tool. The aim is to show any residues that are realistically binding to each ligand. 2D pictures show the tensions in the binding pouch of receptors that stabilise ligands to examine the drug liquidity of 19 tannins, all molecular properties to be the inhibitor candidate for COVID-19 were further elucidated (Liu 2009; Macchiagodena et al. 2020).
Molecular dynamics simulations and pharmacophore analysis
Molecular modelling computations have been conducted with the purpose of refinement of our docking results and analysis of the assemblage performance and constant use of the SARS-CoV-2 3CLpro crystal model for the top three hydrolysable tannins Software GROMOS was used to simulate 80 ns MD after the similar technique as previously mentioned CHARMM force field was used for the ligand topology files via CGenFF server. The octahedron box with the water model TIP3P solved the ligand–protein complexes. The projected pedunculagin pharmacophore has also been developed.
Discussion
Examination of the allosteric connection of 19 distinct structural tannins with protease COVID-19 initially indicated several positions and the best sachets with modest scores were utilized. Compatible molecules, previously used by the co-crystallized inhibitor, are reiterated to dock In a study of the physicochemical properties in SARS–CoV-2–3CLpro, the protein was identified as a stable hydrophilic molecule capable of making H-bonds with another ligand and consisted of 306 chain-type residues of a polypeptide with molecular weights of 33,796 kDa. Furthermore, the comparison of the SARS-CoV-2-3CLpro protein sequence collected with SARS-like bat reveals a sequence of 99.02% individuality. In addition (Moorthy et al. 2020), it was observed that SARS-CoV-2 is much like SARS than MERS and communicates with bat coronaviruses a mutual ancestor. Further, the results showed that Cis-His (Cys145 and His51) was Cys-His catalytic dyad, reliable with HS-3CLpro (Cys145 and His41), TGEV-3CLpro (Cys144 and His41), and HCoV-3CLpro (SARS-145 and His41) (Cys144 and His41). SARS-CoV-2-3CLpro has been reported to provide a concise and similar receptor binding configuration to SARS-3CLpro binding compact and to improve the chance of SARS-3CLpro predicted inhibitors likewise mitigating SARS-CoV-2-3CLpro movement (Kim et al. 2020).
Research is now increasingly focused on the finding of computer drugs to handle the continued importance of a new and operative tiny molecule as natural therapies against COVID-19 with negligible adverse effects. Several investigations have shown that tannins can be antivirals by computerised medicine methods (Jiang et al. 2020). Hence, all three natural compounds show antiviral characteristics due to their molecular structure and receptors binding sites. Here are some common structures of Alkaloids, Terpenes, Tannins etc. (Table 1).
Future directions
Secondary metabolites extracted from many plants are helpful for both humans and animals. Studies of a number of these alkaloids have shown that many are active in pharmacology. These include cancer, bacterial antibodies, antiviral agents, antioxidants, and antifungals (Gao et al. 2020). As a pandemic infection with health problems has become SARS-CoV-2, the scientist’s community has worked hard to identify possible therapeutic targets for tackling the virus infection. Several articles have recently been released, mainly based on the natural screening of viral target protein compounds (Poduri et al. 2020; Peele et al. 2020; Verma et al. 2020). Computer-assisted approaches are useful tools for identifying prospective novel medications, particularly in emerging disorders such as COVID-19. By using breakthrough drug design tools in the field of organic chemistry, potential molecules have been produced that can be used to identify various goals of different coronaviruses. Computational techniques such as docking, Density functional theory (DFT), and molecular simulations have been examined in several chemical entities already; nevertheless, no new compounds, others for repurposed medicines, have been offered until now (Yang et al. 2006). Taking this into account, drug repurposing represents a successful strategy to combat delicate diseases in the future. Combined with polypharmacology, a novel therapeutic model could be the possibility to achieve the helpful natural drug scaffold against SARS-CoV-2 objectives (Silva Antonio et al. 2020).
Conclusion
In drug discovery till today, NP has played an active and vital role. Their use as antiviral drugs is, however, limited and increasing constantly. Our research of the bibliographies of NPs against HCoV shows that there are numerous options for more complete treatment and examination of current NPs to identify new antivirals. Detected products tend to rotate around relatively common and not very diversified characters as revealed in the literature survey NP databases and the library of NP with samples accessible for bioactivity screening may minimise the time necessary for the compound to achieve bioactivity evaluation. The available literature is limited in terms of anti-HCoV activity and NPs, to draw sound findings concerning structure–activity linkages. Even so, the findings suggest that alkaloids, triterpene saponins, and tannins are in trend. Research on HCoVs and NPs has until now been fragmented, due to emergencies like the SARS-CoV emergence in 2002 and the present SARS-CoV-2 danger. While there are several published results, the systemic study in NPs has not shown promising activity, and research attempts to define the mechanism of actions of these compounds must address this problem. In this area, a multidisciplinary approach is required to develop composites based on the greater power scaffold, more advantageous physical–chemical characteristics and reduced toxicity. NPs offer a widening chemical diversity, particularly as a result of the current pressure of this global epidemic.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus
- 3CL-pro:
-
3C-Like protease
- ACE2:
-
Angiotensin-converting enzyme
- CoV:
-
Coronavirus
- MERS:
-
Middle East respiratory syndrome
- NP:
-
Natural products
- HCMV:
-
Human cytomegalovirus
- HCV:
-
Hepatitis C Virus
- HIV:
-
Human immunodeficiency virus
- ZV:
-
Zika virus
- HSV1:
-
Herpes simplex virus type 1
- CQ:
-
Quinine
- HCQ:
-
Hydroxychloroquine
- MRC-5:
-
Medical research cell strain
- PEDV:
-
Porcine epidemic diarrhea virus
- JEV:
-
Japanese encephalitis virus
- MHVs:
-
Mice hepatitis virus
- TGEV:
-
Transmissible gastroenteritis virus
- MEP:
-
Methylerythritol phosphates
- DMAPP:
-
Dimethylallyl diphosphate
- MVA:
-
Melavonic acid
- IPP:
-
Isopentenyl diphosphates
- THC:
-
Tetrahydrocannabinol
- GABA:
-
Gamma aminobutyric acid
- GDP:
-
Geranyl diphosphate
- CBD:
-
Cannabidiol
- CBGA:
-
Cannabigerolic acid
- OA:
-
Olevetolic acid
- FDP:
-
Farnesyl diphosphate
- Aldh1:
-
Aldehyde dehydrogenase 1
- DBR2:
-
Double bond reductase
- SERCA:
-
Sarcoendoplasmic reticulum calcium transport ATPase
- FDA:
-
Food and drug administration
- CBD:
-
Cannabidiol
- OD:
-
Ocular thickness
- EBV:
-
Epstein-Barr virus
- EG:
-
Ellagic acid
- RoG:
-
Gyrator radius
- DFT:
-
Density functional theory
References
Andre CM, Hausman JF, Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 7:1–17
Arato Ferreira PH, dos Santos DAP, da Silva MFDG, Vieira PC, King-Diaz B, Lotina-Hennsen B, Veiga TAM (2016) Acridone alkaloids from Swinglea glutinosa (Rutaceae) and their effects on photosynthesis. Chem Biodivers 13:100–106
Astani A, Reichling J, Schnitzler P (2010) Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 24(5):673–679
Astani A, Schnitzler P (2014) Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J Microbiol 6:149–155
Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW (2006) Patapoutian, A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 9:493
Barrett ML, Scutt AM, Evans FJ (1986) Cannflavin A and B, prenylated flavones from Cannabis sativa L. Experientia 42:452–453
Berry M, Fielding B, Gamieldien J (2015) Potential broad spectrum inhibitors of the coronavirus 3CLpro: a virtual screening and structure-based drug design study. Viruses 7:6642–6660
Brook K, Bennett J, Desai SP (2017) The chemical history of morphine: an 8000-year journey, from resin to de-novo synthesis. J Anesth Hist 3:50–55
Bulduk, Gezer B, Cengiz M. (2015) Optimization of Ultrasound-Assisted Extraction of Morphine from Capsules of Papaver somniferum by Response Surface Methodology. Int J Anal Chem 20151–20158
Buzzini P, Arapitsas P, Goretti M, Branda E, Turchetti B, Pinelli P, Romani A (2008) Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev Med Chem 8(12):1179
Cao J, Forrest JC, Zhang X (2015) A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res 114:1–10
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816
Chang J, Cho JH, Lee KHH, Lee KP, Han MW, Lee SS (1995) Antitumor activity of pedunculagin, one of the ellagitannin. Arch Pharmacal Res 18(6):396
Chang SJ, Chang YC, Lu KZ, Tsou YY, Lin CW (2012) Antiviral activity of Isatis indigotica extract and its derived indirubin against Japanese encephalitis virus. Evid Based Complement Altern Med 2012:1–7
Chen SHA, Desmond JE (2005b) Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia 43(9):1227–1237
Chen QB, Gao J, Zou GA, Xin XL (2018a) A piperidine alkaloids with diverse skeletons from Anacyclus pyrethrum. J Nat Prod 81:1474–1482
Chen L-C, Zhu Y, Papandreou G, Schroff F, Adam H (2018b) Encoderdecoder with atrous separable convolution for semantic image segmentation. In Proceedings of the European conference on computer vision (ECCV), pp 801–818
Chen LR, Wang YC, Lin YW, Chou SY, Chen SF, Liu LT, Juang SH (2005a) Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg Med Chem Lett 15:3058–3062
Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, Luo R et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:125–34
Cheng F, Wu J, Fang L, Wang X (2012) Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Frontiers Plant Sci 3198
Cheng (2020) Covid-19: risk factors for severe disease and death. Bmj 368
Choudhary S, Malik YS, Tomar S (2020) IdentificationofSARS-CoV-2cell entry inhibitors by drug repurposing using in silico structure based virtual screening approach. Front Immunol 11:1664
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 361:2045–2046
Cronemberger Sebastião, Nassim Calixto, Marcelo Nacif Moraes, Iuri David Castro, Patrícia Cordeiro Lana, and Artur Furst Loredo. (2012) Efficacy of one drop of 2% pilocarpine to reverse the intraocular pressure peak at 6:00 am in early glaucoma. Vision Pan-America 11(1):14
Cushnie TPT, Cushnie B, Lamb AJ (2014) Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents 44:377–386
da Silva AA, Wiedemann LSM, Veiga-Junior VF (2020) Natural products’ role against COVID-19. RSC Adv 10:23379–23393
Dai J-P, Wang Q-W, Su Y, Gu L-M, Deng H-X, Chen X-X, Li W-Z, Li K-S (2018) Oxymatrine inhibits influenza A virus replication and inflammation via TLR4, p38 MAPK and NF-κB pathways. Int J Mol Sci 19(4):965
Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J et al (2017) Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global Health 5(12):e1192–e1207
Diosa-Toro M, Troost B, van de Pol D, Heberle AM, Urcuqui-Inchima S, Thedieck K, Smit JM (2019) Tomatidine, a novel antiviral compound towards dengue virus. Antivir Res 161:90–99
Elzinga S, Fischedick J, Podkolinski R, Raber JC (2015) Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat Prod Chem Res 3:10–4172
Ero I, Abu Eida EY, Csóka I, Sánta Z, Cserne A, Kövé T (2003) Optimization of drug release from dermatological semisolid preparations. Drug Dev Res 59:316–325
Fellermeier M, Zenk MH (1998) Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett 427(2):283–285
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14(1):101–115
Flores-Sanchez IJ, Verpoorte R (2008) Secondary metabolism in cannabis. Phytochem Rev 7:615–639
Forman SB, Pariser DM, Poulin Y, Vincent MS et al (2020) TYK2/JAK1 inhibitor PF-06700841 in patients with plaque psoriasis: Phase IIa, randomized, double-blind, placebo controlled Trial. J Investig Dermatol 140:2359-2370.e5
Formukong EA, Evans AT, Evans FJ (1998) Analgesic and anti inflammatory activity of constituents of Cannabis sativa L. Inflammation 12:361–371
Fossati E, Narcross L, Ekins A, Martin Falgueyret J P (2015) Synthesis of morphinan alkaloids in Saccharomyces cerevisiae. PLoS ONE 10:e0124459
Friedman D, Devinsky O (2015) Cannabinoids in the treatment of epilepsy. N Engl J Med 373:1048–1058
Funck-Brentano C, Salem J (2020) Funck-Brentano, C, Salem J-E (2020) RETRACTED: Chloroquine or hydroxychloroquine for COVID-19: why might they be hazardous? The Lancet 2020
Gagne SJ, Stout JM, Liu E, Boubakir Z, Page CSM (2012) Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc Natl Acad Sci USA 109:12811–12816
Galeotti N, Mannelli LDC, Mazzanti G, Bartolini A, Ghelardini C (2002) Menthol: a natural analgesic compound. Neurosci Lett 322:145–148
Gao J, Tian Z, Yang X (2020) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72–73
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, T Dupont, Honor´e S, Colson P, Chabri`ere E, La Scola B, Rolain JM, Brouqui P and D Raoul (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56(1):105949
Grof CPL (2018) Cannabis, from plant to pill. Br J Clin Pharmacol 84:2463–2467
Gu W, Wang W, Li X, Zhang Y, Wang L, Yuan C, Huang L, Hao X (2015) A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia. Fitoterapia 107:60–62
Guerrero-García C, Rubio-Guerra AF (2018) Combination therapy in the treatment of hypertension. Drugs Context 7:212531
Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R (2019) Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 24:2471
Gulland JMR, Mem R (1952) Constitution of codeine and thebaine. Proc Manch Lit Phil Soc 69:79-86.6
Gupta A, Bhati V, Kumar A (2021) Herbals for COVID-19 infection: COVID-19 Curr Chall Future Perspect 1: 160
Gutzeit HO, Ludwig-Müller J (2008) PlantNaturalProducts: Synthesis, Biological Functions and Practical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. 105. Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: ∆9-tetrahydrocannabinol, cannabidiol and ∆9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215
Gyebi GA, Adegunloye AP, Ibrahim IM, Ogunyemi OM, Afolabi SO, Ogunro OB (2022) Prevention of SARS-CoV-2 cell entry: insight from in silico interaction of drug-like alkaloids with spike glycoprotein, human ACE2, and TMPRSS2. J Biomol Struct Dyn 40(5):2121–2145
Gyuris JM, Forgo P, Molnár J, Hohmann J (2004) Alkaloids from Leucojum vernum and antiretroviral activity of Amaryllidaceae alkaloids. Planta Medica 70:09871–873
Hazekamp A, Tejkalová K, Papadimitriou S (2016) Cannabis: from cultivar to chemovar II—a metabolomics approach to Cannabis classification. Cannabis Cannabinoid Res 1:202–215
He LJ, Liu JS, Luo D, Zheng YR, Zhang YB, Wang GC, Li YL (2019) Quinolizidine alkaloids from Sophora tonkinensis and their anti-inflammatory activities. Fitoterapia. 139:104391
He FG, Martinowich K, Chin MH, Fouse FSD, Hutnick L, Hattori C et al. (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling 3345-3356
Heinig U, Aharoni A (2014) Analysis of Steroidal Alkaloids and Saponins in Solanaceae Plant Extracts Using UPLC-qTOF Mass Spectrometry. Humana Press, New York, NY, USA, pp 171–185
Hensel H (1951) Zotterman Y (1951) The effect of menthol on the thermoreceptors. Acta Physiol Scand 24:27–34
Hensel H, Zotterman Y (1951) Action potentials of cold fibres and intracutaneous temperature gradient. J Neurophys 14(5):377–385
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. 87:1932–1936
Ho YJ, Lu JW, Huang YL, Lai ZZ (2019) Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem Biophys Res Commun 518:732–738
Hung TC, Jassey A, Liu CH, Lin CJ, Lin CC, Wong SH, Wang JY, Yen MH (2019) Lin L T (2019) Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 53:62–69
Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z (2020) Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med 35:1545–1549
Jo S, Kim H, Kim S, Shin DH, Kim MS (2019) Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des 94(6):2023–2030
Kang K, Ming B, G, Kim G J, Choi H, Oh W K, Sung S H, (2015) Jubanines F-J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry 119:90–95. https://doi.org/10.1016/j.phytochem.2015.09.001
Kaplan BL, Springs AE (2008) Kaminski N E (2008) The profile of immunemodulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol 76:726–737
Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S (2020) Potential inhibitor of COVID-19 main Protease (Mpro) from several medicinal plant compounds by molecular docking study. Prepr 1:1–14
Khan IU, Sajid S, Javed A, Sajid S, Shah SU (2017) Comparative diagnosis of typhoid fever by polymerase chain reaction and widal test in Southern Districts (Bannu, Lakki Marwat and DI Khan) of Khyber Pakhtunkhwa. Pakistan. Acta Sci Malaysia 1(2):12–15
Khan FA, Maalik A (2015) Advances in pharmacology of isatin and its derivatives: a review. Trop J Pharm Res 14:1937–1942
Kim DE, Min JS, Jang MS, Lee JY, Shin YS, Park CM, Jin YH (2019) Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules 9:696
Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181(4):914–921
Kishimoto S, Sato M, Tsunematsu Y, Watanabe K (2016) Evaluation of biosynthetic pathway and engineered biosynthesis of alkaloids. Molecules 21:1078
Kuo YC, Kuo YH, Lin YL, Tsai WJ (2006) Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression. Antiviral Res 70:112–120
Lau BK, Karim S, Goodchild AK, Vaughan CW, Drew GM (2014) Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br J Pharmacol 171:2803–2813
Lee YZ, Yang CW, Hsu HY, Qiu YQ, Yeh TK, Chang HY, Chao YS, Lee SJ (2012) Synthesis and biological evaluation of tylophorine-derived dibenzoquinolines as orally active agents: exploration of the role of tylophorine E ring on biological activity. J Med Chem 55:10363–10377
Li SY, Chen C, Zhang Q, Guo HY, Wang H, Wang L, Xiao PG (2005) Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67:18–23
Lin CW, Tsai FJ, Tsai CH, Lai CC, Wan L, Ho TY, Hsieh CC, Chao PDL (2005) Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir Res 68:36–42
Lin LT, Chen TY, Chung CY, Noyce RS, Grindley TB, Mccormick C, Richardson CD (2011) Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. Virol 85(9):4386–4398
Lin (1997) Spectrosomes and Fusomes Anchor Mitotic Spindles during Asymmetric Germ Cell Divisions and Facilitate the Formation of a Polarized Microtubule Array for Oocyte Specification inDrosophila. Dev Biol 189(1):79–94
Lin J, Qiu S, Lewis K, Klibanov AM (2002) Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol Prog 18:1082–1086
Liu GT (2009) Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med Chem 5:29–43
Liu W, Zhu HM, Niu GJ, Shi EZ, Chen J, Sun B, Yang C (2014) Synthesis, modification and docking studies of 5-sulfonyl isatin derivatives as SARS-CoV 3C-like protease inhibitors. Bioorg Med Chem 22:292–302
Liu C, Cai D, Zhang L, Tang W, Yan R, Guo H, Chen X (2016) Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Res 134:97–107
Liu W, Junbo Chen Z, Xiang R, Song H, Shu S, Chen L et al (2020) Detection of Covid-19 in children in early January 2020 in Wuhan. China. N Engl J Med 382(14):1370–1371
Luganini A, Mercorelli B, Messa L, Palù G, Gribaudo G (2019) Loregian A (2019) The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antivir Res 164:52–60
Macchiagodena M, Pagliai M, Procacci P (2020) Identification of potential binders of the main protease 3CLpro of the COVID-19 via structure-based ligand design and molecular modeling. Chem Phys Lett 750137489
Maehle AH (2009) (2009) A binding question: the evolution of the receptor concept. Endeavour 33:135–140
Maier H J, Bickerton E, Britton P (2015) Coronaviruses Methods and Protocols; Humana Press: Heidelberg, Germany. pp. 1–282. ISBN 9781493924387.
Manayi A, Nabavi SM, Setzer WN, Jafari S (2019) Piperine as a potential anti-cancer agent: a review on preclinical studies. Curr. Med. Chem. 25:4918–4928
Martin NJ, Ferreiro SF, Barbault F, Lecellier NM, Paetz G, Gaysinski C, Alonso M, Thomas E, Botana OP (2020) Indole alkaloids from the Marquesan plant Rauvolfia nukuhivensis and their effects on ion channels. Phytochemistry 109:84–95
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC (1990) Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561
McMahon JB, Currens MJ, Gulakowski RJ, Buckheit RW, Lackman-Smith C, Hallock YF, Boyd MR (1995) Michellamine B, a novel plant alkaloid, inhibits human immunodeficiency virus-induced cell killing by at least two distinct mechanisms. Antimicrob Agents Chemother 39:484–488
De Meijer E, Hammond K (2020) Distribution of chemical phenotypes (Chemotypes) in European agricultural hemp (Cannabis sativa L.) cultivars. J Forensic Sci 65(3):715–721
Moorthy V, Restrepo AMH, Preziosi MP, Swaminathan S (2020) Data sharing for novel coronavirus (COVID-19). Bull World Health Organ 98(3):150
Moradi MT, Karimi A, Rafieian Kopaei M, Fotouhi F (2017) In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb Pathog 110:42–49
Nakagawa A, Minami H, Kim JS, Koyanagi T, Katayama T, Sato F, Kumagai H (2011) A bacterial platform for fermentative production of plant alkaloids. Nat Commun 2:326
Oliveira SL, da Silva MS, Tavares JF, Sena-Filho JG, Lucena HFS, Romero MAV, Barbosa-Filho JM (2010) Alkaloids from Erythroxylum genus: distribution and compilation of 13C-NMR spectral data. Chem Biodivers 7:302–326
Page KM, Suarez-Farinas M, Suprun M, Zhang W, Garcet S, Fuentes-Duculan J, Li X, Scaramozza M, Kieras E, Banfield C (2020) Molecular and cellular responses to the TYK2/JAK1 inhibitor PF-06700841 reveal reduction of skin in-flammation in plaque psoriasis. J Investig Dermatol 140:1546-1555.e4
Page SW, Maddison JE (2008) Principles of clinical pharmacology. Small Animal Clinical Pharmacology 2:1–26
Peele KA, Chandrasai P, Srihansa T, Krupanidhi S, Sai AV, Babu DJ, Indira M, Reddy AR, Venkateswarulu T (2020) Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: a computational study. Inform Med Unlocked 19:100345
Pham LV, Ngo HTT, Lim YS (2012) Hwang, S.B. Hepatitis C virus non-structural 5B protein interacts with cyclin A2 and regulates viral propagation. J Hepatol 57:960–966
Pillaiyar T, Meenakshisundaram S, Manickam M (2020) Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today 25(4):668–688
Pleschka S, Stein M, Schoop R (2009) Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and Swine-Origin H1N1 (S-OIV). Virol J 6:197
Poduri R, Joshi G, Jagadeesh G (2020) Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell Signal 74:109721
Qamar MT, Alqahtani SM, Alamri MA, Chen L-L (2020) Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal 10(4):313–319
Qin G, Xu R (1998) Recent advances on bioactive natural products from Chinese medicinal plants. Med Res Rev 18:375–382
Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Mackay CR (1998) The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig 101(4):746–754
Rea KA, Casaretto JA, Al-Abdul-Wahid MS, Sukumaran A, Geddes-McAlister J, Rothstein SJ, Akhtar TA (2019) Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry 164:162–171
Ricardo-da-Silva López-Pérez PM, Elvira C, Mano JF, Román JS, Reis RL (2008) Poly (Nisopropylacrylamide) surface-grafted chitosan membranes as a new substrate for cell sheet engineering and manipulation. Biotech Bioeng 101:1321–1331
Richins RD, Rodriguez-Uribe L, Lowe K, Ferral R, Connell O, M A, (2018) Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS ONE 13:e0201119
Rogowska-Szadkowska D, Strumiło J, Chlabicz S (2018) Is medical marijuana legalisation possible in Poland? Cent Eur J Public Health 26:45
Rui X, Tan H, Yan Q (2014) Nanostructured metalsulfides for energy storage. Nanoscale 6(17):9889–9924
Rui C, Yuxiang L, Ning J, Ningtian M, Qingluan Z, Yinju H, Ru Z, Lin M, Tao S, Jianqiang Y (2013) Anti-apoptotic and neuroprotective effects of oxysophoridine on cerebral ischemia both in vivo and in vitro. Planta Med 79:916–923
Salehi B, Sharopov F, Tumer TB, Ozleyen A, Rodríguez-Pérez C, Ezzat SM, Azzini E, Hosseinabadi T, Butnariu M, Sarac I (2019) Symphytum species: a comprehensive review on chemical composition, food applications and phytopharmacology. Molecules 24:2272
Sepúlveda L, Ascacio A, Rodríguez-Herrera R, Aguilera-Carbό A, Aguilar CN (1999) Processing of cellular ceramics by foaming and in situ polymerisation of organic monomers. J Eur Ceram Soc 19:2059–2066
Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura Calixto F (2009) Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 53(S2):S310–S329
Shahni R, Handique PJ (2013) Antibacterial properties of leaf extracts of Strobilanthes cusia (Nees) Kuntze, a rare ethno-medicinal plant of Manipur. India Int J Pharmtech Res 5:1281–1285
Shen L, Niu J, Wang C, Huang B, Wang W, Zhu N, Cen S (2019) High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol 93:e00023-e19
Shoyama Y, Hirano H (1984) Nishioka I (1984) Biosynthesis of propyl cannabinoid acid and its biosynthetic relationship with pentyl and methyl cannabinoid acids. Phytochemistry 23:1909–1912
Silva RM, Pereira LD, Véras JH, Do Vale CR, Chen-Chen L, Da Costa SS (2016) Protective effect and induction of DNA repair by Myrciaria cauliflora seed extract and pedunculagin on cyclophosphamide -induced genotoxicity. Mutation Res/Gen Toxicol Environ Mutagenesis 810:40–47
Silva Antônio AMd (2020) On the possibility of interrupting the coronavirus (COVID-19) epidemic based on the best available scientific evidence. Revista Brasileira de Epidemiologia 23:e200021
Simre M, Tack J (2018) Analysis of risk factors and prevention strategies for functional delayed gastric emptying in 1243 patients with distal gastric cancer. World J Surg Oncol 18:11–10
Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Wada Y, Shoyama Y, Taura F (2004) The gene controlling marijuana psychoactivity: molecular cloning and heterologous expression of D1-tetrahydrocannabinolic acid synthase from Cannabis Sativa L. J Biol Chem 279:39767–39774
Smeriglio A, Barreca D, Bellocco E, Trombetta D (2017) Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. British J Pharmacol 174(11):1244–1262
Smeriglio A, Trombetta D, Laganà G (2020) Colored phytonutrients: role and applications in the functional foods of anthocyanins. In Phytonutrients in Food. Woodhead Publishing, pp 177–195
Solymosi K, Kofalvi A (2017) Cannabis: a treasure trove or pandora’s box? Mini Rev Med Chem 17:1223–1291
Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M (2006) Modulation of cytokine expression by traditional medicines: are view of herbal immunomodulators. Altern Med Rev 11:128–150
Tasnim S, Hossain MM, Mazunder H (2020) J Prev Med Public Health 2020(53):171–174
Taura F, Morimoto S, Shoyama Y, Mechoulam R (1995) First direct evidence for the mechanism of. DELTA. 1-tetrahydrocannabinolic acid biosynthesis. J Am Chem Soc 117:9766–9767
Taura F, Sirikantaramas S, Shoyama Y, Yoshikai K, Shoyama Y, Morimoto S (2007) Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett 581:2929–2934
Taura F, Tanaka S, Taguchi C, Fukamizu T, Tanaka H, Shoyama Y, Morimoto S (2009) Characterization of olivetol synthase, a polyketide synthase reputative involved in cannabinoid biosynthetic pathway. Febslett 583:22
Thanacoody R (2016) Quinine and chloroquine. Medicine 44(3):197–198
Thompson PL, Nidorf SM (2018) Colchicine: an affordable anti-inflammatory agent for atherosclerosis. Curr Opin Lipidol 29:467–473
Turner JC, Hemphill JK, Mahlberg PG (1978) Quantitative determination of cannabinoids in individual glandular trichomes of Cannabis sativa L. (Cannabaceae). Am J Bot 65:1103–1106
Uozaki M, Yamasaki H, Katsuyama Y, Higuchi M, Higuti T, Koyama AH (1998) 2 D NMR in α−(h 8− B E D T− T T F) 2 ND 4 Hg (SCN) 4. Phys Rev B 57(22):14422
Ushio Y, Fang T, Okuda T, Abe H (2012) L type Ca2+ channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Molecular Pain 8:1744–8069
Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567
Varghese FS, Thaa B, Amrun SN, Simarmata D, Rausalu K, Nyman TA, Merits A, McInerney GM, Ng LFP, Ahola T (2016) The antiviral alkaloid Berberine reduces Chikungunya virus-induced mitogen-activated protein kinase signaling. J Virol 90:9743–9757
Verma S, Twilley D, Esmear T, Oosthuizen CB, Reid AM, Nel M, Lall N (2020) Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19). Front Pharmacol 11:561334
Voets T, Owsianik G, Janssens A, Talavera K, Nilius B (2007) TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 3:174
Wang T, Liu X, Guan J, Ge S, Wu MB, Lin J, Yang L (2019) Advancement of multi-target drug discoveries and promising applications in the field of Alzheimer’s disease. Eur J Med Chem 169:200–223
Webber SE, Tikhe J, Worland ST, Fuhrman SA, Hendrickson TF, Matthews DA, Ferre RA (1996) Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J Med Chem 39:5072–5082
Weber C, Opatz T (2019) Bisbenzylisoquinoline alkaloids. Alkaloids. Chem Biol 81:1–114
Werz O, Seegers J, Schaible AM, Weinigel C, Barz D, Koeberle A, Allegrone G, Pollastro F, Zampieri L, Grassi G (2014) Cannflavins from hemp sprouts, novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. Pharma Nutrition 2:53–60
Xu W, Zhang M, Liu H, Wei K, He M, Li X, Hu D, Yang S, Zheng Y (2019) Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx. Nat Prod Res 33:1486–1490
Yang H, Yang M, Ding Y, Liu Y, Lou Z, Zhou Z, Rao Z (2003) The crystal structures of severe acute respiratory syndrome virus main protease andits complex with an inhibitor. Proc Natl Acad Sci 100(23):13190–13195
Yang H, Bartlam M, Rao Z (2006) Drug design targeting the main protease, the Achilles heel of coronaviruses. Curr Pharm Des 12:4573–4590
Yang CW, Lee YZ, Kang IJ, Barnard DL, Jan JT, Lin D, Lee SJ (2010) Identification of phenanthroindolizines and phenanthroquinolizidines as novel potent anti-coronaviral agents for porcine enteropathogenic coronavirus transmissible gastroenteritis virus and human severe acute respiratory syndrome coronavirus. Antiviral Res 88:160–168
Yang C, Lee W, Hsu YZ, Shih HY, Chao YS, Chang HY, Lee SJ (2017) Targeting coronaviral replication and cellular JAK2 mediated dominant NF-κB activation for comprehensive and ultimate inhibition of coronaviral activity. Sci Reports 7:1–13
Yang H, Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ (2004) Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods 289(1–2):211–223
Yao X, Zhang Y, Long W, Liu P (2012) Oxysophoridine suppresses the growth of hepatocellular carcinoma in mice. In Vivo and cDNA microarray studies. Chin J Integr Med 18:209–213
Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, Lee S (2018) Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359:237–241
Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, Sato Y et al (2011) Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478(736):64–69
Yu X, Gao X, Zhu Z, Cao Y, Zhang Q, Tu P, Chai X (2014a) Alkaloids from the Tribe Bocconieae (Papaveraceae): a chemical and biological review. Molecules 19:13042–13060
Yu X, Lu B, Xu Z (2014b) Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4–3D graphene hybrid electrodes. Advanced materials 26(7):1044–1051
Zeng G, Zhang J, Chen Y, Yu Z, Yu M, Li H, Liu Z, Chen M, Lu L, Hu C (2011) Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting. J Immunol Methods 102(19):9026–9032
Zhang H, Cisse M, Dauphin YN, Lopez-Paz D (2017) mixup: Beyond empirical risk minimization. arXiv preprint arXiv:1710.09412
Zhang YN, Zhang QY, Li XD, Xiong J, Xiao SQ, Wang Z, Zhang ZR, Deng CL, Yang XL (2020) Wei H P (2020) Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg Microbes Infect 9:1170–1173
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Hao-Rusi S, Zhu Y, Li B, Huang CL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579:270–273
Zuo Y, Lin A, Chang P, Gan WB (2005) Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46(2):181–189
Zhu Y, Papandreou G, Schroff F, Adam H (2018) Encoder-decoder with atrous separable convolution for semantic image segmentation. In Proceedings of the European conference on computer vision (ECCV), pp. 801–818
Author information
Authors and Affiliations
Contributions
AAH, TB: Conceived the idea and wrote the draft; TU, SC, SB, SS and NS: Literature Review; AS, SV, VRP, SD and RK: Figure Work; LA and SBU: Proof Read.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have approved and reviewed the current manuscript for final publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Harrasi, A., Behl, T., Upadhyay, T. et al. Targeting natural products against SARS-CoV-2. Environ Sci Pollut Res 29, 42404–42432 (2022). https://doi.org/10.1007/s11356-022-19770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19770-2