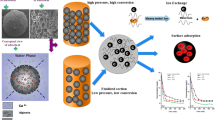
Abstract
This research aimed to develop a novel composite as a low-cost adsorbent for the removal of nitrate ion from aqueous solutions. The characterization of this composite (composition of red mud with dimethyldioctadecylammonium bromide (DDAB)) was performed by XRF, XRD, FTIR, and BET analyses. The most influential variables on nitrate adsorption, including contact time, solution acidity, adsorbent amount, and temperature were studied. The maximum amount of nitrate adsorbed onto the prepared adsorbent was obtained at pH 5.5 and contact time 30 min. The heterogeneous adsorption occurred during the uptake of nitrate. The results of kinetic study revealed that intra-particle diffusion was the major limitation for nitrate adsorption rate. The values of thermodynamic parameters illustrate the non-spontaneous, associative, and exothermic adsorption process. Increasing the temperature enhances the tendency of the process to non-spontaneously. Research on fixed-bed column has been done under different initial nitrate concentrations. The adsorption capacity of nitrate was increased with an increase in the initial concentration of nitrate. The results of column data were successfully explained using the Thomas and Yoon–Nelson models.













Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Abou-Shady A, Peng C, Bi J, Xu H, Almeria OJ (2012) Recovery of Pb (II) and removal of NO3− from aqueous solutions using integrated electrodialysis, electrolysis, and adsorption process. Desalination 286:304–315
Adesemuyi MF, Adebayo MA, Akinola AO, Olasehinde EF, Adewole KA, Lajide L (2020) Preparation and characterisation of biochars from elephant grass and their utilisation for aqueous nitrate removal: effect of pyrolysis temperature. J Environ Chem Eng 8:104507
Akhurst DJ, Jones GB, Clark M, McConchie D (2006) Phosphate removal from aqueous solutions using neutralised bauxite refinery residues (Bauxsol™). Environ Chem 3:65–74
Alagha O, Manzar MS, Zubair M, Anil I, Mu’azu ND, Qureshi A, (2020) Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: insight into behavior and mechanisms. Nanomaterials 10:1361
Allahkarami E, Igder A, Fazlavi A, Rezai B (2017) Prediction of Co (II) and Ni (II) ions removal from wastewater using artificial neural network and multiple regression models. Physicochem Probl Miner Process 53:1105–1118
Allahkarami E, Soleimanpour Moghadam N, Jamrotbe B, Azadmehr A (2021) Competitive adsorption of Ni (II) and Cu (II) ions from aqueous solution by vermiculite-alginate composite: batch and fixed-bed column studies. J Dispers Sci Technol 1:1–11
Allahkarami E, Rezai B (2019) Removal of cerium from different aqueous solutions using different adsorbents: a review. Process Saf Environ Prot 124:345–362
Almughamisi MS, Khan ZA, Alshitari W, Elwakeel KZ (2020) Recovery of chromium(VI) oxyanions from aqueous solution using Cu(OH)2 and CuO embedded chitosan adsorbents. J Polym Environ 28:47–60
Baei MS, Esfandian H, Nesheli AA (2016) Removal of nitrate from aqueous solutions in batch systems using activated perlite: an application of response surface methodology. Asia Pac J Chem Eng 11:437–447
Barber WP, Stuckey DC (2000) Nitrogen removal in a modified anaerobic baffled reactor (ABR): 1, denitrification. Water Res 34:2413–2422
Battas A, Gaidoumi AE, Ksakas A, Kherbeche A (2019) Adsorption study for the removal of nitrate from water using local clay. Sci World J 2019:9529618
Bhatnagar A, Kumar E, Sillanpää M (2010) Nitrate removal from water by nano-alumina: characterization and sorption studies. Chem Eng J 163:317–323
Camargo JA, Alonso Á (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32:831–849
Cengeloglu Y, Tor A, Ersoz M, Arslan G (2006) Removal of nitrate from aqueous solution by using red mud. Sep Purif Technol 51:374–378
Chatterjee S, Lee MW, Woo SH (2009) Influence of impregnation of chitosan beads with cetyl trimethyl ammonium bromide on their structure and adsorption of congo red from aqueous solutions. Chem Eng J 155:254–259
Chiu H-F, Tsai S-S, Yang C-Y (2007) Nitrate in drinking water and risk of death from bladder cancer: an ecological case-control study in Taiwan. J Toxicol Environ Health A 70:1000–1004
Deihimi N, Irannajad M, Rezai B (2018) Characterization studies of red mud modification processes as adsorbent for enhancing ferricyanide removal. J Environ Manage 206:266–275
Despland LM, Clark MW, Vancov T, Aragno M (2014) Nutrient removal and microbial communities’ development in a young unplanted constructed wetland using Bauxsol™ pellets to treat wastewater. Sci Total Environ 484:167–175
Dimas Rivera GL, Martínez Hernández A, Pérez Cabello AF, Rivas Barragán EL, Liñán Montes A, Flores Escamilla GA, Sandoval Rangel L, Suarez Vazquez SI, De Haro Del Río DA (2021) Removal of chromate anions and immobilization using surfactant-modified zeolites. J Water Process Eng 39:101717
El-Korashy SA, Elwakeel KZ, Abd El-Hafeiz A (2016) Fabrication of bentonite/thiourea-formaldehyde composite material for Pb (II), Mn (VII) and Cr (VI) sorption: a combined basic study and industrial application. J Clean Prod 137:40–50
El-Safty SA, Shenashen MA (2013) Optical mesosensor for capturing of Fe(III) and Hg(II) ions from water and physiological fluids. Sensors Actuators B Chem 183:58–70
El-Safty SA, Ismael M, Shahat A, Shenashen MA (2013) Mesoporous hexagonal and cubic aluminosilica adsorbents for toxic nitroanilines from water. Environ Sci Pollut Res 20:3863–3876
Elwakeel KZ, Al-Bogami AS (2018) Influence of Mo(VI) immobilization and temperature on As(V) sorption onto magnetic separable poly p-phenylenediamine-thiourea-formaldehyde polymer. J Hazard Mater 342:335–346
Elwakeel KZ, Elgarahy AM, Khan ZA, Almughamisi MS, Al-Bogami AS (2020) Perspectives regarding metal/mineral-incorporating materials for water purification: with special focus on Cr (vi) removal. Mater Adv 1:1546–1574
Eriksson LGT, Claesson PM, Eriksson JC, Yaminsky VV (1996) Equilibrium wetting studies of cationic surfactant adsorption on mica: 1. Mono-and bilayer adsorption of CTAB. J Colloid Interface Sci 181:476–489
Erol B, Erol K, Gökmeşe E (2019) The effect of the chelator characteristics on insulin adsorption in immobilized metal affinity chromatography. Process Biochem 83:104–113
Erol K, Bolat M, Tatar D, Nigiz C, Köse DA (2020) Synthesis, characterization and antibacterial application of silver nanoparticle embedded composite cryogels. J Mol Struct 1200:127060
Fernandes AN, Almeida CAP, Menezes CTB, Debacher NA, Sierra MMD (2007) Removal of methylene blue from aqueous solution by peat. J Hazard Mater 144:412–419
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect 112:1371–1374
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Golestanifar H, Asadi A, Alinezhad A, Haybati B, Vosoughi M (2016) Isotherm and kinetic studies on the adsorption of nitrate onto nanoalumina and iron-modified pumice. Desalin Water Treat 57:5480–5487
Gomaa H, Shenashen MA, Yamaguchi H, Alamoudi AS, Abdelmottaleb M, Cheira MF, Seaf El-Naser TA, El-Safty SA (2018) Highly-efficient removal of AsV, Pb2+, Fe3+, and Al3+ pollutants from water using hierarchical, microscopic TiO2 and TiOF2 adsorbents through batch and fixed-bed columnar techniques. J Clean Prod 182:910–925
Gomaa H, Shenashen MA, Elbaz A, Kawada S, Seaf El-Nasr TA, Cheira MF, Eid AI, El-Safty SA (2021a) Inorganic-organic mesoporous hybrid segregators for selective and sensitive extraction of precious elements from urban mining. J Colloid Interface Sci 604:61–79
Gomaa H, Shenashen MA, Elbaz A, Yamaguchi H, Abdelmottaleb M, El-Safty SA (2021b) Mesoscopic engineering materials for visual detection and selective removal of copper ions from drinking and waste water sources. J Hazard Mater 406:124314
Günay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371
Hamdaoui O (2006) Dynamic sorption of methylene blue by cedar sawdust and crushed brick in fixed bed columns. J Hazard Mater 138:293–303
He H, Huang Y, Yan M, Xie Y, Li Y (2020) Synergistic effect of electrostatic adsorption and ion exchange for efficient removal of nitrate. Colloids Surf A Physicochem Eng Aspects 584:123973
Hu H-Y, Goto N, Fujie K (2001) Effect of pH on the reduction of nitrite in water by metallic iron. Water Res 35:2789–2793
Hu Q, Chen N, Feng C, Hu W (2015) Nitrate adsorption from aqueous solution using granular chitosan-Fe3+ complex. Appl Surf Sci 347:1–9
Huang W, Wang S, Zhu Z, Li L, Yao X, Rudolph V, Haghseresht F (2008) Phosphate removal from wastewater using red mud. J Hazard Mater 158:35–42
Igder A, Fazlavi A, Allahkarami E, Dehghanipour A (2019) Optimization of Ni(II) & Co(II) removal from wastewater and statistical studies on the results of experimental designs. Geosyst Eng 22:91–100
Jain S, Bansiwal A, Biniwale RB, Milmille S, Das S, Tiwari S, Siluvai Antony P (2015) Enhancing adsorption of nitrate using metal impregnated alumina. J Environ Chem Eng 3:2342–2349
Kireç O, Alacabey İ, Erol K, Alkan H (2021) Removal of 17β-estradiol from aqueous systems with hydrophobic microspheres. J Polym Eng 41:226–234
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Li Y, Liu C, Luan Z, Peng X, Zhu C, Chen Z, Zhang Z, Fan J, Jia Z (2006) Phosphate removal from aqueous solutions using raw and activated red mud and fly ash. J Hazard Mater 137:374–383
Li D, Ding Y, Li L, Chang Z, Rao Z, Lu L (2015) Removal of hexavalent chromium by using red mud activated with cetyltrimethylammonium bromide. Environ Technol 36:1084–1090
Lin S-H, Juang R-S (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manage 90:1336–1349
Liu A, Ming J, Ankumah RO (2005) Nitrate contamination in private wells in rural Alabama, United States. Sci Total Environ 346:112–120
Liu Q, Xin R, Li C, Xu C, Yang J (2013) Application of red mud as a basic catalyst for biodiesel production. J Environ Sci 25:823–829
Luo W, Huang Q, Zhang X, Antwi P, Mu Y, Zhang M, Xing J, Chen H, Ren S (2020) Lanthanum/Gemini surfactant-modified montmorillonite for simultaneous removal of phosphate and nitrate from aqueous solution. J Water Process Eng 33:101036
Namasivayam C, Sangeetha D (2005) Removal and recovery of nitrate from water by ZnCl2 activated carbon from coconut coir pith, an agricultural solid waste. Indian J Chem Technol 12:513–521
Öztürk N, Bektaş TEl (2004) Nitrate removal from aqueous solution by adsorption onto various materials. J Hazard Mater 112:155–162
Paledi U, Allahkarami E, Rezai B, Aslani MR (2021) Selectivity index and separation efficiency prediction in industrial magnetic separation process using a hybrid neural genetic algorithm. SN Appl Sci 3:351
Rai S, Wasewar K, Mukhopadhyay J, Yoo CK, Uslu H (2012) Neutralization and utilization of red mud for its better waste management. World 6:5410
Ren Z, Xu X, Wang X, Gao B, Yue Q, Song W, Zhang L, Wang H (2016) FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr (VI) removal by amine cross-linking biosorbent. J Colloid Interface Sci 468:313–323
Rezai B, Allahkarami E (2021a) Chapter 2 - wastewater treatment processes—techniques, technologies, challenges faced, and alternative solutions. In: Karri RR, Ravindran G, Dehghani MH (eds) Soft Computing Techniques in Solid Waste and Wastewater Management. Elsevier, Amsterdam, pp 35–53
Rezai B, Allahkarami E (2021b) Chapter 4 - application of neural networks in wastewater degradation process for the prediction of removal efficiency of pollutants. In: Karri RR, Ravindran G, Dehghani MH (eds) Soft Computing Techniques in Solid Waste and Wastewater Management. Elsevier, Amsterdam, pp 75–93
Santona L, Castaldi P, Melis P (2006) Evaluation of the interaction mechanisms between red muds and heavy metals. J Hazard Mater 136:324–329
Schoeman JJ, Steyn A (2003) Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 155:15–26
Sepahvand P, Abdizadeh GR, Noori S (2020) Inverse design of an irregular-shaped radiant furnace using neural network and a modified hybrid optimization algorithm. Therm Sci Eng Prog 20:100730
Sepehri S, Heidarpour M, Abedi-Koupai J (2014) Nitrate removal from aqueous solution using natural zeolite-supported zero-valent iron nanoparticles. Soil Water Res 9:224–232
Shittu I, Achazhiyath Edathil A, Alsaeedi A, Al-Asheh S, Polychronopoulou K, Banat F (2019) Development of novel surfactant functionalized porous graphitic carbon as an efficient adsorbent for the removal of methylene blue dye from aqueous solutions. J Water Process Eng 28:69–81
Soares MIM (2000) Biological denitrification of groundwater. In: Belkin S (ed) Environmental Challenges. Springer Netherlands, Dordrecht, pp 183–193
Song W, Gao B, Xu X, Wang F, Xue N, Sun S, Song W, Jia R (2016) Adsorption of nitrate from aqueous solution by magnetic amine-crosslinked biopolymer based corn stalk and its chemical regeneration property. J Hazard Mater 304:280–290
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327
Ugurlu M (2009) Adsorption studies and removal of nitrate from bleached kraft mill effluent by fly-ash and sepiolite. Fresenius Environ Bull 18:2328–2335
Wan D, Liu H, Liu R, Qu J, Li S, Zhang J (2012) Adsorption of nitrate and nitrite from aqueous solution onto calcined (Mg–Al) hydrotalcite of different Mg/Al ratio. Chem Eng J 195–196:241–247
Wang S, Ang HM, Tadé MO (2008) Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72:1621–1635
World Health Organization (2011) Guidelines for drinking-water quality. World Health Organization 216:303–304
Wu F-C, Liu B-L, Wu K-T, Tseng R-L (2010) A new linear form analysis of Redlich-Peterson isotherm equation for the adsorptions of dyes. Chem Eng J 162:21–27
Zhan Y, Lin J, Zhu Z (2011) Removal of nitrate from aqueous solution using cetylpyridinium bromide (CPB) modified zeolite as adsorbent. J Hazard Mater 186:1972–1978
Zhao Y, Wang J, Luan Z, Peng X, Liang Z, Shi L (2009) Removal of phosphate from aqueous solution by red mud using a factorial design. J Hazard Mater 165:1193–1199
Zulfadhly Z, Mashitah M, Bhatia S (2001) Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ Pollut 112:463–470
Author information
Authors and Affiliations
Contributions
S.F. did the batch experiments, and S.F. and F.N. interpreted the results of batch experiments. A.A. managed the project. E.A., F.N., and A.A. wrote the manuscript. E.A. did the experiments for continuous adsorption process and interpreted them. E.A, A.A. and M.S. participated in the interpretation of results, review, and editing of the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Allahkarami, E., Azadmehr, A., Noroozi, F. et al. Nitrate adsorption onto surface-modified red mud in batch and fixed-bed column systems: equilibrium, kinetic, and thermodynamic studies. Environ Sci Pollut Res 29, 48438–48452 (2022). https://doi.org/10.1007/s11356-022-19311-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19311-x