Abstract
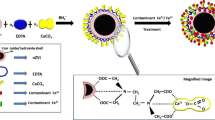
Calcium ion-incorporated hydrous iron(III) oxide (CIHIO) samples have been prepared aiming investigation of efficiency enhancement on arsenic and fluoride adsorption of hydrous iron(III) oxide (HIO). Characterization of the optimized product with various analytical tools confirms that CIHIO is microcrystalline and mesoporous (pore width, 26.97 Å; pore diameter, 27.742 Å with pore volume 0.18 cm3 g−1) material. Increase of the BET surface area (> 60%) of CIHIO (269.61 m2 g−1) relative to HIO (165.6 m2 g−1) is noticeable. CIHIO particles are estimated to be ~ 50 nm from AFM and TEM analyses. Although the pH optimized for arsenite and fluoride adsorptions are different, the efficiencies of CIHIO towards their adsorption are very good at pH 6.5 (pHzpc). The adsorption kinetics and equilibrium data of either tested species agree well, respectively, with pseudo-second order model and Langmuir monolayer adsorption phenomenon. Langmuir capacities (mg g−1at 303 K) estimated are 29.07 and 25.57, respectively, for arsenite and fluoride. The spontaneity of adsorption reactions (ΔG0 = − 18.02 to − 20.12 kJ mol−1 for arsenite; − 0.2523 to − 3.352 kJ mol−1 for fluoride) are the consequence of entropy parameter. The phosphate ion (1 mM) compared to others influenced adversely the arsenite and/or fluoride adsorption reactions. CIHIO (2.0 g L−1) is capable to abstract arsenite or fluoride above 90% from their solution (0 to 5.0 mg L−1). Mechanism assessment revealed that the adsorption of arsenite occurs via chelation, while of fluoride occurs with ion-exchange.











Similar content being viewed by others
References
Addo Ntim S, Mitra S (2012) Adsorption of arsenic on multiwall carbon nanotube–zirconia nanohybrid for potential drinking water purification. J Colloid Interface Sci 375:154–159. https://doi.org/10.1016/j.jcis.2012.01.063
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091. https://doi.org/10.1021/cr300133d
Babel S (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243. https://doi.org/10.1016/S0304-3894(02)00263-7
Babic BM, Milonjic JM, Kaludierovic VB (1999) Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon 37:477–481
Basu T, Ghosh UC (2011) Arsenic(III) removal performances in the absence/presence of groundwater occurring ions of agglomerated Fe(III)–Al(III) mixed oxide nanoparticles. J Ind Eng Chem 17:834–844. https://doi.org/10.1016/j.jiec.2011.09.002
Basu T, Gupta K, Ghosh UC (2010) Equilibrium and thermodynamics on arsenic(III) sorption reaction in the presence of background ions occurring in groundwater with nanoparticle agglomerates of hydrous iron(III) + chromium(III) mixed oxide †. J Chem Eng Data 55:2039–2047. https://doi.org/10.1021/je901010x
Biswas K, Bandhoyapadhyay D, Ghosh UC (2007a) Adsorption kinetics of fluoride on iron(III)-zirconium(IV) hybrid oxide. Adsorption 13:83–94. https://doi.org/10.1007/s10450-007-9000-1
Biswas K, Saha SK, Ghosh UC (2007b) Adsorption of fluoride from aqueous solution by a synthetic iron(III)−Aluminum(III) mixed oxide. Ind Eng Chem Res 46:5346–5356. https://doi.org/10.1021/ie061401b
Biswas K, Gupta K, Ghosh UC (2009) Adsorption of fluoride by hydrous iron(III)–tin(IV) bimetal mixed oxide from the aqueous solutions. Chem Eng J 149:196–206. https://doi.org/10.1016/j.cej.2008.09.047
Biswas K, Debnath S, Ghosh UC (2010) Physicochemical aspects on fluoride adsorption for removal from water by synthetic hydrous iron(III) – chromium(III) mixed oxide. Sep Sci Technol 45:472–485. https://doi.org/10.1080/01496390903526667
Cadaval TRS Jr, Dotto GL, Pinto LAA (2015) Equilibrium isotherms, thermodynamics and kinetic studies for the adsorption of food azo dyes onto chitosan films. Chem Eng Commun 202:1316–1323. https://doi.org/10.1080/00986445.2014.934449
Cao C-Y, Cui Z-M, Chen C-Q et al (2010) Ceria hollow nanospheres produced by a template-free microwave-assisted hydrothermal method for heavy metal ion removal and catalysis. J Phys Chem C 114:9865–9870. https://doi.org/10.1021/jp101553x
Chandra V, Park J, Chun Y et al (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4:3979–3986. https://doi.org/10.1021/nn1008897
Chaturvedi AK, Yadava KP, Pathak KC, Singh VN (1990) Defluoridation of water by adsorption on fly ash. Water Air Soil Pollut 49:51–61. https://doi.org/10.1007/BF00279509
Chen L, He B-Y, He S et al (2012) Fe―Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technol 227:3–8. https://doi.org/10.1016/j.powtec.2011.11.030
Clesceri LS, American Public Health Association, American Water Works Association, Water Pollution Control Federation (eds) (1998) Standard methods: for the examination of water and wastewater, 20th. edn. American Public Health Ass, Washington
Crittenden JC, Borchardt JH, Harza MW (eds) (2012) MWH’s water treatment: principles and design, 3rd. edn. Wiley, Hoboken
Deschamps E, Ciminelli VST, Höll WH (2005) Removal of As(III) and As(V) from water using a natural Fe and Mn enriched sample. Water Res 39:5212–5220. https://doi.org/10.1016/j.watres.2005.10.007
Dey S, Goswami S, Ghosh UC (2004) Hydrous ferric oxide (HFO)—a scavenger for fluoride from contaminated water. Water Air Soil Pollut 158:311–323. https://doi.org/10.1023/B:WATE.0000044854.71497.b6
Fallahzadeh RA, Miri M, Taghavi M et al (2018) Spatial variation and probabilistic risk assessment of exposure to fluoride in drinking water. Food Chem Toxicol 113:314–321. https://doi.org/10.1016/j.fct.2018.02.001
Fan X (2003) Adsorption kinetics of fluoride on low cost materials. Water Res 37:4929–4937. https://doi.org/10.1016/j.watres.2003.08.014
Fornaro T, Burini D, Biczysko M, Barone V (2015) Hydrogen-bonding effects on infrared spectra from anharmonic computations: uracil–water complexes and uracil dimers. J Phys Chem A 119:4224–4236. https://doi.org/10.1021/acs.jpca.5b01561
Frantz TS, Silveira N Jr, Quadro MS, Andreazza R, Barcelos AA, Cadaval TRS Jr, Pinto LAA (2017) Cu(II) adsorption from copper mine water by chitosan films and the matrix effects. Environ Sci Pollut Res 24:5908–5917. https://doi.org/10.1007/s11356-016-8344-z
Ghosh UC, Bandyopadhyay D, Manna B, Mandal M (2006) Hydrous Iron(III)-Tin(IV) Binary mixed oxide: arsenic adsorption behaviour from aqueous solution. Water Quality Research Journal 41:198–209. https://doi.org/10.2166/wqrj.2006.023
Ghosh A, Chakrabarti S, Biswas K, Ghosh UC (2014) Agglomerated nanoparticles of hydrous Ce(IV)+Zr(IV) mixed oxide: preparation, characterization and physicochemical aspects on fluoride adsorption. Appl Surf Sci 307:665–676. https://doi.org/10.1016/j.apsusc.2014.04.095
Gupta VK, Ali I, Saini VK (2007) Defluoridation of wastewaters using waste carbon slurry. Water Res 41:3307–3316. https://doi.org/10.1016/j.watres.2007.04.029
Gupta K, Biswas K, Ghosh UC (2008) Nanostructure iron(III)−zirconium(IV) binary mixed oxide: synthesis, characterization, and physicochemical aspects of arsenic(III) sorption from the aqueous solution. Ind Eng Chem Res 47:9903–9912. https://doi.org/10.1021/ie8002107
Gupta K, Maity A, Ghosh UC (2010) Manganese associated nanoparticles agglomerate of iron(III) oxide: synthesis, characterization and arsenic(III) sorption behavior with mechanism. J Hazard Mater 184:832–842. https://doi.org/10.1016/j.jhazmat.2010.08.117
Gupta VK, Ali I, Saleh TA et al (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv 2:6380. https://doi.org/10.1039/c2ra20340e
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223. https://doi.org/10.1021/i160018a011
Jing C, Cui J, Huang Y, Li A (2012) Fabrication, characterization, and application of a composite adsorbent for simultaneous removal of arsenic and fluoride. ACS Appl Mater Interfaces 4:714–720. https://doi.org/10.1021/am2013322
Ku Y, Chiou H-M (2002) The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut 133:349–361. https://doi.org/10.1023/A:1012929900113
Kumar E, Bhatnagar A, Ji M et al (2009) Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res 43:490–498. https://doi.org/10.1016/j.watres.2008.10.031
Lai YD, Liu JC (1996) Fluoride removal from water with spent catalyst. Sep Sci Technol 31:2791–2803. https://doi.org/10.1080/01496399608000827
Li Z, Deng S, Yu G et al (2010) As(V) and As(III) removal from water by a Ce–Ti oxide adsorbent: behavior and mechanism. Chem Eng J 161:106–113. https://doi.org/10.1016/j.cej.2010.04.039
Li H, Li W, Zhang Y et al (2011a) Chrysanthemum-like α-FeOOH microspheres produced by a simple green method and their outstanding ability in heavy metal ion removal. J Mater Chem 21:7878. https://doi.org/10.1039/c1jm10979k
Li W, Cao C-Y, Wu L-Y et al (2011b) Superb fluoride and arsenic removal performance of highly ordered mesoporous aluminas. J Hazard Mater 198:143–150. https://doi.org/10.1016/j.jhazmat.2011.10.025
Li M, Wang C, O’Connell MJ, Chan CK (2015) Carbon nanosphere adsorbents for removal of arsenate and selenate from water. Environ Sci Nano 2:245–250. https://doi.org/10.1039/C4EN00204K
Li W, Chen D, Xia F et al (2016) Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environ Sci Nano 3:94–106. https://doi.org/10.1039/C5EN00171D
Li J, Gyoten H, Sonoda A et al (2017) Removal of trace arsenic to below drinking water standards using a Mn–Fe binary oxide. RSC Adv 7:1490–1497. https://doi.org/10.1039/C6RA26806D
Liu H, Deng S, Li Z et al (2010) Preparation of Al–Ce hybrid adsorbent and its application for defluoridation of drinking water. J Hazard Mater 179:424–430. https://doi.org/10.1016/j.jhazmat.2010.03.021
Maliyekkal SM, Sharma AK, Philip L (2006) Manganese-oxide-coated alumina: a promising sorbent for defluoridation of water. Water Res 40:3497–3506. https://doi.org/10.1016/j.watres.2006.08.007
Manna BR, Dey S, Debnath S, Ghosh UC (2003) Removal of arsenic from groundwater using crystalline hydrous ferric oxide (CHFO). Water Qual Res J Can 38:193–210. https://doi.org/10.2166/wqrj.2003.013
Mayadevi S (1996) Adsorbents for the removal of fluoride from water. Ind Chem Engg Sect A 38:155–157
Medellin-Castillo NA, Leyva-Ramos R, Ocampo-Perez R et al (2007) Adsorption of fluoride from water solution on bone char. Ind Eng Chem Res 46:9205–9212. https://doi.org/10.1021/ie070023n
Milonlic SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72(12):1363–1367. https://doi.org/10.2298/JSC0712363M
Miri M, Bhatnagar A, Mahdavi Y et al (2018) Probabilistic risk assessment of exposure to fluoride in most consumed brands of tea in the Middle East. Food Chem Toxicol 115:267–272. https://doi.org/10.1016/j.fct.2018.03.023
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Mukhopadhyay K, Ghosh A, Das SK et al (2017) Synthesis and characterisation of cerium( iv )-incorporated hydrous iron( iii ) oxide as an adsorbent for fluoride removal from water. RSC Adv 7:26037–26051. https://doi.org/10.1039/C7RA00265C
Patel G, Pal U, Menon S (2009) Removal of fluoride from aqueous solution by CaO nanoparticles. Sep Sci Technol 44:2806–2826. https://doi.org/10.1080/01496390903014425
Paul B, Parashar V, Mishra A (2015) Graphene in the Fe 3 O 4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ Sci: Water Res Technol 1:77–83. https://doi.org/10.1039/C4EW00034J
Pendergast MM, Hoek EMV (2011) A review of water treatment membrane nanotechnologies. Energy Environ Sci 4:1946. https://doi.org/10.1039/c0ee00541j
Raichur A, Jyoti Basu M (2001) Adsorption of fluoride onto mixed rare earth oxides. Sep Purif Technol 24:121–127. https://doi.org/10.1016/S1383-5866(00)00219-7
Rongshu W, Haiming L, Ping N, Ying W (1995) Study of a new adsorbent for fluoride removal from waters. Water Qual Res J Can 30:81–88. https://doi.org/10.2166/wqrj.1995.012
Saha I, Gupta K, Chakraborty S et al (2014) Synthesis, characterization and As(III) adsorption behavior of β-cyclodextrin modified hydrous ferric oxide. J Ind Eng Chem 20:1741–1751. https://doi.org/10.1016/j.jiec.2013.08.026
Saha I, Ghosh A, Nandi D et al (2015) β-Cyclodextrin modified hydrous zirconium oxide: Synthesis, characterization and defluoridation performance from aqueous solution. Chem Eng J 263:220–230. https://doi.org/10.1016/j.cej.2014.11.039
Saha I, Kanrar S, Gupta K et al (2016) Tuned synthesis and characterizational insight into β-cyclodextrin amended hydrous iron-zirconium hybrid oxide: a promising scavenger of fluoride in aqueous solution. RSC Adv 6:93842–93854. https://doi.org/10.1039/C6RA16567B
Sanchooli Moghaddam M, Rahdar S, Taghavi M (2016) Cadmium removal from aqueous solutions using saxaul tree ash. Iran J Chem Chem Eng 35:8
Siddiqui AH (1955) Fluorosis in Nalgonda District, Hyderabad-Deccan. Br Med J 2:1408–1413
Sivasamy A, Singh KP, Mohan D, Maruthamuthu M (2001) Studies on defluoridation of water by coal-based sorbents. J Chem Technol Biotechnol 76:717–722. https://doi.org/10.1002/jctb.440
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568. https://doi.org/10.1016/S0883-2927(02)00018-5
Taghavi M, Ehrampoush MH, Ghaneian MT et al (2018a) Application of a Keggin-type heteropoly acid on supporting nanoparticles in photocatalytic degradation of organic pollutants in aqueous solutions. J Clean Prod 197:1447–1453. https://doi.org/10.1016/j.jclepro.2018.06.280
Taghavi M, Ghaneian TM, Ehrampoush HM, Tabatabaee M, Afsharnia M, Alami A, J M (2018b) Feasibility of applying the LED-UV-induced TiO2/ZnO-supported H3PMo12O40 nanoparticles in photo catalytic degradation of aniline. Environ Monit Assess 190:188. https://doi.org/10.1007/s10661-018-6565-y
Tian Y, Wu M, Liu R et al (2011) Modified native cellulose fibers—a novel efficient adsorbent for both fluoride and arsenic. J Hazard Mater 185:93–100. https://doi.org/10.1016/j.jhazmat.2010.09.001
Viswanathan N, Sundaram CS, Meenakshi S (2009) Sorption behaviour of fluoride on carboxylated cross-linked chitosan beads. Colloids Surf B: Biointerfaces 68:48–54. https://doi.org/10.1016/j.colsurfb.2008.09.009
Wang B, Wu H, Yu L et al (2012) Template-free formation of uniform urchin-like α -FeOOH hollow spheres with superior capability for water treatment. Adv Mater 24:1111–1116. https://doi.org/10.1002/adma.201104599
Wang S, Gao B, Zimmerman AR et al (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395. https://doi.org/10.1016/j.biortech.2014.10.104
World Health Organization (ed) (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Yang X, Wang X, Feng Y et al (2013) Removal of multifold heavy metal contaminations in drinking water by porous magnetic Fe 2 O 3 @AlO(OH) superstructure. J Mater Chem A 1:473–477. https://doi.org/10.1039/C2TA00594H
Yang J, Zhang H, Yu M et al (2014) High-content, well-dispersed γ-Fe 2 O 3 nanoparticles encapsulated in macroporous silica with superior arsenic removal performance. Adv Funct Mater 24:1354–1363. https://doi.org/10.1002/adfm.201302561
Yavuz CT, Mayo JT, Yu WW et al (2006) Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314:964–967. https://doi.org/10.1126/science.1131475
Yu X-Y, Xu R-X, Gao C et al (2012) Novel 3D hierarchical cotton-candy-like CuO: surfactant-free solvothermal synthesis and application in As(III) removal. ACS Appl Mater Interfaces 4:1954–1962. https://doi.org/10.1021/am201663d
Zhong L-S, Hu J-S, Liang H-P et al (2006) Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv Mater 18:2426–2431. https://doi.org/10.1002/adma.200600504
Zhong L-S, Hu J-S, Wan L-J, Song W-G (2008) Facile synthesis of nanoporous anatase spheres and their environmental applications. Chem Commun:1184. https://doi.org/10.1039/b718300c
Acknowledgement
The authors are grateful to the Principal, Maharaja Manindra Chandra College for providing laboratory facilities, and Presidency University for extending some research facilities. One of the authors (KB) is thankful to UGC for the financial support [F.PSW-087/15-16 (ERO)] of this work, and PS is thankful to W.B. State DHESTBT [211(Sanc.)/ST/P/S&T/15G-14/2017] for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOCX 70 kb)
Rights and permissions
About this article
Cite this article
Ghosh, A., Paul, S., Bhattacharya, S. et al. Calcium ion incorporated hydrous iron(III) oxide: synthesis, characterization, and property exploitation towards water remediation from arsenite and fluoride. Environ Sci Pollut Res 26, 4618–4632 (2019). https://doi.org/10.1007/s11356-018-3872-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3872-3