Abstract
Recently, it was observed that there is an increasing application of nanoparticles (NPs) in aquaculture. Manufacturers are trying to use nano-based tools to remove the barriers about waterborne food, growth, reproduction, and culturing of species, their health, and water treatment in order to increase aquaculture production rates, being the safe-by-design approach still unapplied. We reviewed the applications of NPs in aquaculture evidencing that the way NPs are applied can be very different: some are direclty added to feed, other to water media or in aquaculture facilities. Traditional toxicity data cannot be easily used to infer on aquaculture mainly considering short-term exposure scenarios, underestimating the potential exposure of aquacultured species. The main outputs are (i) biological models are not recurrent, and in the case, testing protocols are frequently different; (ii) most data derived from toxicity studies are not specifically designed on aquaculture needs, thus contact time, exposure concentrations, and other ancillary conditions do not meet the required standard for aquaculture; (iii) short-term exposure periods are investigated mainly on species of indirect aquaculture interest, while shrimp and fish as final consumers in aquaculture plants are underinvestigated (scarce or unknown data on trophic chain transfer of NPs): little information is available about the amount of NPs accumulated within marketed organisms; (iv) how NPs present in the packaging of aquacultured products can affect their quality remained substantially unexplored. NPs in aquaculture are a challenging topic that must be developed in the near future to assure human health and environmental safety.

ᅟ
Similar content being viewed by others
Introduction
Aquaculture and fisheries supply about 15% of the average animal protein consumption to 2.9 billion people worldwide in, and is still increasing. Approximately 43.5 million people are directly employed within these sectors, and 520 million people indirectly derive their livelihoods from aquaculture and fisheries industries (Asche et al. 2015).
Similarly, nanotechnology is no more a niche for researchers, but a really fast growing and impacting key economical field providing new nanoenabled products with novel and unique functions. The new-engineered nanoenabled products, improved by nanoparticles (NPs), have been the key factor for the success of the nanotechnology industry. With a size between 1 and 100 nm on at least one dimension, NPs present unique physico-chemical properties that differ from their bulk materials, such as a greater surface area to volume ratio, resulting in a larger reactivity. Due to their remarkable properties, NPs have been widely used in different fields such as energy and electronics, wastewater treatment, personal care products, and medicine and agriculture (ETC 2003; Karnik et al. 2005; Aitken et al. 2006; Libralato et al. 2013; Callegaro et al. 2015; Dasgupta et al. 2015; Perera et al. 2015; Libralato 2014; Libralato et al. 2016a; Minetto et al. 2014, 2016; Podyacheva and Ismagilov 2015; Vale et al. 2016). Recently, nanotechnology has found several applications in aquaculture, but their implications are still unknown.
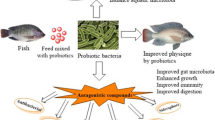
In the fishery and aquaculture industry, NPs are used for several direct and indirect applications as summarized in Fig. 1. Indirect uses include water and wastewater treatment, fishpond sterilization, and harvested fish packaging for commercialization like as barcoding and tagging; direct uses involve feeding industry and animal healthcare like fish disease control.
The escalating production and application of NPs have raised concerns about their safety to human health and the environment. While a significant number of studies have been conducted on NP potential toxicity toward humans and other organisms, few have been directed toward the effects in aquaculture. The assessment of potential bioadverse effects of NPs would allow the determination of a safe limit concentration to be used on food production activities such as fishery and aquaculture. Moreover, this could trigger the discussion on the regulatory use of NPs in the food industry and the creation of proper legislation, which are still currently missing.
The present study reviewed for the first time the potential toxicity of NPs in aquaculture providing a critical summary of recent scientific literature on their potential hazardous effects. Our focus is not the environment, but aquacultured species intentionally treated with NPs or indirectly exposed to NPs used in aquaculture activities.
Aquaculture industry and nanotechnology
Nanotechnology and aquatic feed
One of the most important nanotechnology application in aquaculture is the feed production where the use of NPs have proved to be effective for (i) micronutrient delivery (e.g., chitosan NPs), (ii) amount of produced feed per unit time (e.g., single-walled carbon nanotubes (SWCNTs), fullerenes (C60), and nTiO2), and (iii) growth promotion (e.g., nFe, nSe, nTiO2, and nZnO) (Table 1).
Chitosan [poly(1,4-β-D-glucopyranosamine)] is a polysaccharide with low immunogenicity, low toxicity, and antimicrobial potential being widely used on feed production for human and animals (Rather et al. 2013; Luo and Wang 2013; Ferosekhan et al. 2014; Vendramini et al. 2016). Novel applications of chitosan NPs, for the delivery of unstable and/or hydrosoluble micronutrients, are in early stages of development. Alishahi et al. (2014) showed that the use of chitosan NPs significantly increased shelf life and delivery of vitamin C in rainbow trout after 20 days of feeding. Jiménez-Fernández et al. (2014) conducted a similar study applying chitosan NPs for delivering ascorbic acid (AA) in (i) zebrafish liver cell line (ZFL) and (ii) in vivo to the rotifer Brachionus plicatilis. NPs had the ability to penetrate fish intestinal epithelium showing a significant increase of AA on both models. Rotifers fed with AA-NPs increased up to twofold their AA levels in comparison to the control groups.
During the administration of feed directly to water, nutrients can be relased from feed pellets to water. Chitosan NPs can be used as an encapsulating agent for nutrients that can easily degradate when in contact with water (Chatterjee and Judeh 2016; Ji et al. 2015). Peniche et al. (2004) prevented the leakage of liver oil of shark when encapsulated with calcium alginate coated with chitosan. Klinkesorn and Mcclements (2009) conducted an in vitro study and demonstrated that encapsulation of tuna oil droplets, with chitosan NPs, increased physical stability and subsequently decreased the fatty acids released from the emulsions.
Addition of SWCNTs (Fraser et al. 2011; Bisesi et al. 2015), C60 (Fraser et al. 2011), and nTiO2 (Ramsden et al. 2009) to rainbow trout fathead minnows and rainbow trout food changed the physical properties of fish pellet resulting more compact than usual, decreasing the nutrients’ leaching and their subsequent waste in fishpond.
Selenium (Se) is a trace element essential for life, and has been recently considered in many case studies for animal nutrition (Polettini et al. 2015; Sabbioni et al. 2015). Se is a component of glutathione peroxidase (GSH-Px) enzymes (Rotruck et al. 1973) that protect the cell membrane through glutathione reduction. Supplemental Se can be acquired through diet (Fotedar and Munilkumar 2016; Wang et al. 2013), and Se NPs are gaining a great deal of attention due to its bioavailability and antioxidant defense properties (Sonkusre et al. 2014). Supplemental nSe increased the final weight, protein content in muscle, and GSH-Px activity in liver and blood plasma as well as decreased FCR in crucian carp (Carassius auratus gibelio) that were fed with supplemented diets (Zhou et al. 2009). Additionally, Wang et al. (2013) evidenced that nSe caused an increase in LDH, cellular protein contents, Na+/K+-ATPase, SOD, and GSH-Px in crucian carp (C. auratus gibelio), being this effect both NPs size and dose dependent. Deng and Cheng (2003) reported that nSe promoted a significant effect on the growth of Nile tilapia (Oreochromis niloticus) at moderate (0.5 mg/kg) and high (2.5 mg/kg) doses of Se NPs via spiked feed presenting a weight gain rate of 86.3 ± 4.7 g.
Zinc (Zn) is another essential micronutrient involved in several metabolic pathways and is essential for the regulation of protein synthesis, energy consumption, and as well as vitamin A and lipid metabolism (Muralisankar et al. 2014). Faiz et al. (2015) investigated nZnO as a source of dietary Zn evidencing improved growth and immune response in grass carp (Ctenopharyngodon idella). Muralisankar et al. (2014) showed a significant increase in protein content, antioxidant enzymes activity, and increased weight in freshwater prawn (Macrobrachium rosenbergii) after 90 days feeding with feed improved with nZnO. Bhattacharyya et al. (2015) investigated the use of nanomaterials (NMs) to induce the growth in aquatic species increasing the proportion of nutrients passing across the gut tissue and into the organism rather than passing through the digestive system and excreted partially or totally unused. Ramsden et al. (2009) used nTiO2 to improve growth performance in rainbow trout (Oncorhynchus mykiss).
Nanotechnology and aquatic reproduction
In artificial reproduction of commercial aquatic animal, one of the most common problems is the incomplete vitellogenesis in females leading to failure of the final oocyte maturation and ovulation. To overcome this problem, it is necessary to develop methods for controlling the reproductive process. Chitosan NPs can be used to carry and release in a controlled way endogenous hormone (Pulavendran et al. 2011). Rather et al. (2013) used salmon hormone chitosan-nAu to overcome the problem of the short life of reproductive hormones in blood, thus avoiding the use of multiple injections in order to enhance reproductive efficacy. Results showed that reproductive hormones were present in blood for a longer period in treated organisms and the relative number of eggs and their fertilization rate also significantly increased. Moreover, chitosan nanoconjugated salmon luteinizing hormone-releasing hormone (CsLHRH) increased the expression level of Sox9 transcripts in gonads and steroid hormonal levels in blood of male and female of Clarias batrachus being helpful for proper gonadal development (Bhat et al. 2016).
Nanotechnology and aquacultured species health
Aquaculture industry has experienced great problems with pathogens (bacteria, fungi, and viruses) that were generally controlled with chemical disinfectants and antibiotics (Huang et al. 2015). Shaalan et al. (2016) reviewed the use of NPs as potential antimicrobials, emphasizing on antibiotic-resistant bacteria in fisheries, nanoparticle-based vaccines, and the development of specific and sensitive tool for diagnosis of bacterial, fungal, and viral diseases in aquaculture. Ramya et al. (2014) showed the protective efficacy of a DNA construct containing extra small virus antisense (XSVAS) gene of nodavirus encapsulated with chitosan NPs in M. rosenbergii increasing its survivability. The fish nanomedicine is in its infancy and several gaps about potential adverse effects to target and non-target species still needs to be addressed.
Rapid detection of phatogens in aquatic organisms can be very effective to disease control, but the available methods are time consuming, costly, and might experience some difficulties in pathogen separation and detection. Guo et al. (2016) conducted a study to design an immunomagnetic NP-based microfluidic system to detect Staphylococcus aureus creating a microfluidic chip with indium tin oxide. Results evidenced that sensitivity and specificity of the detection system were the same of the colony counting method, with a whole shorter detection time without colony cultivation.
Due to chitosan antimicrobial properties, several studies investigated its application for seafood packaging (Alishahi et al. 2014; Hosseini et al. 2016). Ramezani et al. (2015) studied the effect of chitosan and chitosan NPs on silver carp (Hypophthalmicthys molitrix) fillets stored at 4 °C, evidencing that chitosan NPs exhibited interesting antimicrobial activity and the ability to inhibit the TVB-N content improving the general storage potentiality of the product.
Disease prevention and control are crucial for aquaculture under an economical and environmental viewpoint. Thus, vaccination plays an important role on large-scale commercial fish farming. Nanoencapsulated vaccines against Listonella anguillarum in Asian carp (Rajeshkumar et al. 2009), white spot syndrome virus (WSSV), and infectious myoncronis virus (IMNV) (i.e., shrimp farming) (Rajeshkumar et al. 2009; Chalamcheria 2015) have been delevoped. Polyanhydride NPs were used for encapsulating and releasing vaccine antigens determing immunization of shrimp via immersion or with feed (Ross et al. 2014). Rajeshkumar et al. (2009) investigated DNA constructed vacinnes based on nanotechnologies to produce immunologic proteins protecting shrimps from WSSV for up to 7 weeks per application. NP-based carriers, like chitosan, alginates, and poly-lactide-co-glycolide acid (PLGA) for vaccine antigens, together with mild inflammatory inducers orally, showing a high level of protection to fish and shellfish with a relative survival rate of up to 85% in cultured shrimp (Rajeshkumar et al. 2009).
In addition, silica-based NPs can be used for drug (i.e., pharmaceuticals or other therapeutics) administration due to its porous structure and ability to incorporate high doses (Strømme et al. 2009). Some authors (García-Rodríguez et al. 2008; Bhattacharyya et al. 2015) evidenced their potential use in aquaculture in the near future.
Silver (Ag) NPs (nAg) are the most investigated multiple mechanism nano-based antibacterial. The release of silver ions (Ag+) and their binding onto bacterial cell membrane proteins lead to cell membrane disruption and to cell death (Lara et al. 2010; Huang et al. 2011). Dananjaya et al. (2016) investigated the antibacterial function of chitosan-Ag nanocomposites (CAgNCs) against fish pathogenic Aliivibrio salmonicida. CAgNCs inhibited A. salmonicida growth indicating minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) at 50 and 100 mg/L, respectively. No effects of CAgNCs were detected to Danio rerio at 12.5 mg/kg of body weight/day (BW/day) as a feed ingredient and Oplegnathus fasciatus testis cells up to 50 mg/L, thus suggesting its potentiality as an antibacterial agent to the control fish pathogenic bacteria.
Further investigations are also necessary about potential side effects of nanotagging and nanobarcoding when applied directly to organisms. The barcode can be detected by the application of nanoscale components such as radio frequency identification (RFID). These tags can hold more information and can be used as a tracking device, monitoring their metabolism or swimming ability. In the processing and export industry, nanobarcoding can be used effectively to observe various aspects of delivery process and management, tracking the source or delivery status of products (Rather et al. 2011).
Nanotechnology and (waste)water treatment in aquaculture
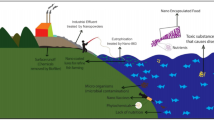
The physico-chemical properties of water in aquaculture ponds can be influenced by various parameters such as soil composition, environmental pollution, and food waste (Venkat 2011; Katuli et al. 2014a, 2014b), while in coastal or open-sea cages, water quality is generally influenced by the natural environment.
Aquatic pollution is one of the greatest threats for aquaculture production. Recently, the application of nanoenabled products based on aerogels, polymers and functionalized composites, hydrophobic organoclays, and magnetic engineered NPs for water treatment and purification has been studied (Bhattacharyya et al. 2015; Lofrano et al. 2016a). nAu, nAg CNTs, nFe, lanthanum (La), and nTiO2 were used for the removal of pesticides, ammonia, heavy metals, and phospahes from water and wastewater (Ren et al. 2011; Xu et al. 2012; Pradeep 2009; Rather et al. 2011). Quantum dots (QDs) due to their unique optical properties (Vázquez-González and Carrillo-Carrion 2014) have been proposed for the detection of heavy metals in aquaculture media (Chen et al. 2013).
Intensive farming of shrimps and fish led to growing problems with bacterial diseases such as A. salmonicida, Flavobacterium columnare, and Yersinia ruckeri (Pulkkinen et al. 2010). In aquaculture, traditional disinfectants (e.g., hydrogen peroxide and malachite green), antibiotics (e.g., sulfonamides and tetracyclines), and anthelmintic agents (e.g., pyrethroid insecticides and avermectins) are frequently used in large amounts, but presenting several limitations like high cost of chemical drugs, negative effects on non-target organisms, and increased resistance of pathogens (Romero et al. 2012).
The proliferation of opportunist pathogens (bacteria, virus, fungi, or protozoa) is a known problem in fish farming due to the high density of organism stocks and the food residues; thus, the use of quick and effective antipathogens is of crucial interest (Twiddy et al. 1995; Castillo-Rodal et al. 2012). For example, nAg was used for the treatment of fungal infections in rainbow trout egg showing inhibitory effect on fungi growth (Johari et al. 2015). nZnO exhibited antibacterial activity disrupting bacterial cell membrane integrity, reducing cell surface hydrophobicity and downregulating the transcription of oxidative stress-resistance genes (Pati et al. 2014). Mühling et al. (2009) showed that nTiO2 and nAg reduced the build-up of bacteria in estuarine water.
The use of Ti photoelectrolysis was used in environmental applications including sterilization and disinfection. Under ultraviolet irradiation conditions, TiO2 NPs produce highly active hydroxyl (OH−), superoxide ion (−O·), and peroxyl radical (O2 −) having high oxidation capacity. Free radicals change cell membrane structure, leading to their apoptosis, thus sterilizing and disinfecting (Yu et al. 2002; Sonawane et al. 2003; Zhao et al. 2000).
Liu et al. (2009) reported bactericidal effects of copper-bearing nanomontmorillonite (Cu2+-MMT) on three aquatic (A. hudrophila, Vibrio parahaemolyticus, and Pseudomonas fluorescens) and two intestinal pathogens (Lactobacillus acidophilus and Bacillus subtilis), showing that the efficency of Cu2+-MMT depended on temperature and contact time. The bacterial removal efficiency was 100% for A. hudrophila, in V. parahaemolyticus, and P. fluorescens, and 24.9% for L. acidophilus and 25.6% in B. subtilis after 12 h at 30 °C.
Wen et al. (2003) stated that the nanodevices are very useful to improve water quality in shrimp aquaculture, reducing the rate of water exchange, improving shrimp survival rate and yield.
Another major challenge in aquaculture is the biofouling control. The bacterial biofilm allows the attachment of macrofoulers, like in the case of mariculture cages causing serious problems like corrosion, weight increase, surface alteration, and distrortion of submerged structures (Champ 2003). To get rid of fouling organisms, antifoulings are directly applied, but with potential undesired adverse effects on other non-target species (e.g., TBT) (Lofrano et al. 2016b). NP-based antifoulings like nCuO, nZnO, and nSi seem to be potential good candidates (Rather et al. 2011) with their high-surface-to-volume ratio creating a more efficient barrier to fouling agents (i.e., at equal or lower concentrations). Ashraf and Edwin (2016) used nCuO to treat cage nets evidencing a significant reduction of fouling after 90 days from application.
“NanoCheck” (Altair Nanotechnologies, Reno, NV, USA) is a commercial product for fishpond management using 40-nm particles based on La compounds supporting the absorption of water phosphates thus limiting algae growth (Mohd Ashraf et al. 2011). Moreover, La oxides NPs were used as phosphate scavenger leading microorganisms to starvation showing promising effects on Escherichia coli, Staphylococcus carnosus, Penicillium roqueforti, and Chlorella vulgaris (Gerber et al. 2012).
Vijayan et al. (2014) assessed the bacterial antibiofilm activity of nAg and nAu synthetized from Turbinaria conoide extracts highlithing that nAg was efficient in controlling biofilm formation, while nAu was not.
Toxicological profiling in tissue-based target
Engineered NPs are applied in various aquaculture sectors, and, currently, many studies are being carried out to check their safe use, but out the aquaculture sector. Since all of their effects on living organisms (especially aquatic organisms) have not been fully identified, public concern raises from their use in aquaculture. Toxicity of NPs can be different in relation to the way they are administered, and toxicokinetics and toxicodynamics. Concentrations of NPs administered via feed, present in treated surfaces (i.e., cage nets), or waterborne (i.e., fishponds) could be significantly higher than the expected NP environmental concentrations (Minetto et al. 2016) being up to micrograms per liter or greater.
We tried to consider a system-based approach, focused on the (eco-)toxicological profile of engineered NPs. In Table 1, we summarized the information related to various NPs and target organisms of potential aquaculture interest, including the main relative testing conditions. NPs were listed and presented considering the following order: alginate, Al2O3, Ag, Au, CeO2, chitosan, chitosan-Ag nanocomposites CAgNCs, CuO, Fe, La, QDs, Se, SiO2, SnO2, SWCNTs and MWCNTs (including C60 and nano carbon black), TiO2, and ZnO. Discussion about the comparison of negative or positive effects of NPs has been very tricky for four main reasons: (i) biological models are not recurrent, and in the case, testing protocols are frequently different; (ii) most data derived from toxicity studies are not specifically designed on aquaculture needs, thus contact time, exposure concentrations, and other ancillary conditions (i.e., acclimation periods) do not meet the required standard for aquaculture; (iii) short-term exposure periods (generally up to 14 days) are investigated mainly on species of indirect aquaculture interest (i.e., A. salina and D. magna as feed for other organisms), while shrimp and fish as final consumers in aquaculture plants are underinvestigated (scarce or unknown data on trophic chain transfer of NPs): little information is available about the amount of NPs accumulated within marketed organisms; and (iv) how NPs present in the packaging of aquacultured products can affect their quality remained substantially unexplored.
Alginate NPs
Alginate is a natural polymer extensively used in food industry as thickening, emulsifying, and stabilizing agent (George and Abraham 2006; Klinkesorn and McClements 2009). Alginate NPs were recently evaluated with positive results (Guo et al. 2013; Guo et al. 2015). However, due to the limited number of toxicity data, concern is present about its use.
Al2O3 nanoparticles
Al2O3 NPs are good dielectric and abrasive agents. Toxicity of nAl2O3 was checked with Caenorhabditis elegans (used as live food in the larval breeding of species in aquaculture and aquaria), showing that concentrations >102 mg/L significantly inhibited the growth and number of eggs inside worm body and offspring, and the worms’ reproduction was inhibited at concentrations >203.9 mg/L of nAl2O3 (Wang et al. 2009).
Shirazi et al. (2015) demostrated that nAl2O3 presented growth inhibition effects on Dunaliella salina, showing a direct relationship between NP concentration and effect. Moreover, the increase in NP concentration corresponded to a chlorophyll and carotenoid decrease in microalgae.
Swain et al. (2014) explored the antimicrobial activity of nAl2O3 (<50 mm) against microbes responsible to diseases in aquaculture. Results showed that nAl2O3 is not able to inhibit the activity of the isolated bacteria.
Barber et al. (2005) exposed nAl2O3 for 72 h to D. rerio, evidencing that ingested NPs were mainly present in the fish intestine and no lethality was recorded up to 500 g/L. Reduced gill ATPase activity was observed, indicating compromised gill function.
Ag NPs
Silver NPs (nAg) are widrespread in several consumer products such as cosmetics and plastics, water purifiers, textiles, drugs, and agrochemicals. Due to their antibacterial activity, nAg has been used in aquaculture for water treatment (Mühling et al. 2009; Johari et al. 2015) and several studies on its toxicity are available on aquatic organisms of aquaculture interest.
Völker et al. (2015) exposed Sphaerium corneum to sub-lethal nAg concentrations (up to 500 μg/L), evidencing a significant ROS generation and antioxidant enzyme activity compared to the control group. Rajkumar et al. (2016) exposed Labeo rohita up to 100 mg/kg of nAg for 7 days, highlighting a significant reduction in hematological parameters. Antioxidant enzymes significantly increased in gills, liver, and muscle; histopathological lesions were evidenced.
Kandasamy et al. (2013) assessed nAgNO3 (synthesized by leaf extract of Prosopis chilensis), showing an antibacterial effect on four species of V. pathogens on shrimps Penaeus monodon after 30 days of exposure. Shrimps fed with nAgNO3 exhibited higher survival rates, associated to immunomodulation in terms of higher hemocyte counts, phenoloxidase, and antibacterial activities of hemolymph. Blinova et al. (2013) studied the adverse effects of nAg to D. magna and Thamnocephalus platyurus. After 24 h of exposure, EC50s of nAg for D. magna and T. platyurus were 17 and 27 μg/L, respectively. According to Arulvasu et al. (2014), Artemia salina was exposed to a series of nAg concentration up to 12 nM for 24–48 h observing that mortality rate, aggregation in gut region, apoptotic cells, and DNA damage increased in a concencetration-dependent way, like cysts hatching rate.
Large-scale culture of Hediste diversicolor provides an increasing market of live baits and can be an important food source for a variety of cultured species like marine prawns or flatfish. García-Alonso et al. (2011) exposed H. diversicolor to nAg@citrate (30 ± 5 nm; 250 ng/g sediment; 10 days), showing aggregations of NPs in close association with the villi, and in the glycolax matrix of the worms’ gut lumen. Cong et al. (2011) investigated H. diversicolor exposed to nAg-spiked sediment, highlighting genotoxicity effects. It is not yet well understood the mechanism of oxidative stress response elicited by nAg and how it relates to the Ag tissue burden. Cozzari et al. (2015) exposed H. diversicolor to sediment spiked with dissolved Ag (added as AgNO3), Ag NPs (63 ± 27 nm), and larger bulk Ag particles (202 ± 56 μm) for up to 11 days at sub-lethal concentrations. Concentration- and time-dependent differences were present in the accumulation of the three Ag forms, but all three forms elicited an oxidative stress response. In the cases of Ag NPs and bulk Ag particles, changes in glutathione, SOD, CAT, GPx, SeGPx, GST, and GR occurred without significant Ag accumulation, while differences in biomarker profiles between the three Ag forms suggest that the mechanism of oxidative stress caused by particulate Ag is distinct from that of dissolved Ag.
Gomes et al. (2013) evaluated the genotoxic impact of nAg using M. galloprovincialis exposed to 10 μg/L of nAg (and its bulk form) for 15 days, assessing genotoxic effects in hemocytes using the comet assay. Ag (nanoparticles and ionic forms) induced DNA damage in hemolymph cells with a time-response effect. Ionic forms presented higher genotoxicity than NPs, suggesting different mechanisms of action that may be mediated through oxidative stress.
Khan et al. (2015) reported on bioaccumulation dynamics in Lumbriculus variegatus of ionic Ag and three differently coated nAg@ (PVP (polyvinylpyrrolidone), PEG (polyethylene glycol), and citrate). Uptake rate constants for nAg were ∼2–10 times less than for Ag+, showing significant rank order concordance with acute toxicity; Ag elimination fitted a 1-compartment loss model.
The effects of AgNPs in Labeo rohita liver were investigated at genomic and cellular level for 7 days at the concentrations of 100, 200, 400, and 800 μg/L (with AgNPs of 18 and 29 nm) (Sharma et al. 2016). After histopathological examination, the liver highlighted vacuolar degeneration, presenting hepatocytes with total degeneration and high accumulation of AgNPs, depicting both time and dose-dependent relationships. Moreover stress-related genes showed downregulation, due to the production of free radicals and reactive oxygen species.
Au NPs
Au NPs (nAu) is used in a variety of fields such as electronics, catalysis, cosmetics, food quality control, and cancer detection (Zhu et al. 2010a, b). Despite its use, little is known about its uptake in aquatic organisms. Asharani et al. (2011) conducted a study to evaluate and compare the effect of Ag, Au, and Pt NPs on the development of zebrafish embryos, evidencing that nAu presented no toxicity compared to nAg (concentration-dependent increase in mortality and phenotypic changes, hatching delays) and nPt (hatching delays).
Mytilus edulis exposed for 24 h to 750 mg/L of Au@citrate NPs highlighted increased CAT activity in the heamolymph, and reduced ubiquitination and caronylation in the digestive gland, gill, and mantle (Tedesco et al. 2008). According to García-Negrete et al. (2013), Ruditapes philippinarum accumulated nAu@citrate (21.5 ± 2.9 nm; 6–30 mg/L) more readily in digestive gland heterolysosomes (plateauing after 12 h), while ionic Au was more associated to gills.
CeO2 nanoparticles
CeO2 NPs are used in coatings, electronics, and biomedical devices and as fuel additives (Falugi et al. 2012). There are still several uncertainties about its effect for human health and the environment. Johnston et al. (2010) exposed D. rerio for 14 days to nCeO2, evidencing Ce accumulation in liver, but not in gill, brain, and skin. A 5-day study (Falugi et al. 2012) investigated the exposure of P. lividus to CeO2 (50–105 nm) NPs at 10 mg/L, resulting in total mortality after only 2 days, but animals survived for 5 days at 0.1 mg/L.
Chitosan NPs
Chitosan is a natural polysaccharide that presents interesting biodegradability (Rather et al. 2013), biocompatibility (Luo and Wang 2013), and mucoadhesiveness (De Campos et al. 2004) properties with potential applications for drug delivery and gene transfer (Chatterjee and Judeh 2016; Ji et al. 2015). Chitosan NPs can pass through tight junctions between epithelial cells (Dodane et al. 1999), posing potential risks to humans, animal, and environment. Hu et al. (2011) reported death and malformation of zebrafish embryos exposed to increasing concentrations of chitosan NPs (200 nm) with almost 100% mortality at 40 mg/L. ROS and hsp70 confirmed that are concentration and size dependent. Rather et al. (2016) studied the effects of kissppetin-10 (K-10) (i.e., an essential gatekeeper of various reproductive processes) and chitosan-encapsulated K-10 nanoparticles (CK-10) on gene expression, evidencing that chitosan nanoparticles increased by 60% the entrapment efficiency for K-10 being potentially useful for developing therapies against various reproductive dysfunctions in vertebrates. Loh et al. (2010) evaluated the cytotoxicity of chitosan NPs in human liver cells showing that CYP3A4 enzyme activity increased in a dose-dependent way. Results highlighted that the destruction of cell membrane was influenced by different zeta potential of chitosan NPs. Similar results were reported by Huang et al. (2004) after exposig A549 cells to chitosan NPs to assess their uptake and cytotoxicity.
Cu NPs
Copper NPs (nCu), especially nCuO, present bactericide and antifouling properties, and an excellent thermal conductivity, being one of the most widely used metallic NPs (Buffet et al. 2011) with potential implications in aquaculture.
Griffitt et al. (2007) exposed D. rerio juveniles for 48 h to waterborne nCuO, observing histological damages, Cu accumulation in gill, and also 82 genes differentially expressed compared to the controls.
Zhao et al. (2011) evaluated the effect of lethal and sub-lethal concentration of nCuO in C. carpio showing that after 4-days exposure, no acute effect was observed, but after a 30-day exposure to sub-lethal concentrations, it was observed a reduced growth and Cu accumulation (intestine > gill > muscle > skin and scale > liver > brain). Moreover, the reduction of cholinesterase activity evidenced that Cu sub-lethal concentrations could have potential neurotoxicity for juveniles.
Buffet et al. (2011) assessed the exposure of H. diversicolor to nCuO (197 nm, 10 μg/L) showing Cu accumulation and oxidative stress evidenced by the increase of GSTs and CAT activities.
Gomes et al. (2013) evaluated the genotoxic impact of nCuO using M. galloprovincialis exposed to 10 μg/L of nCuO (and its bulk form) for 15 days assessing genotoxic effects in hemocytes using the comet assay. Cu (nanoparticles and ionic forms) induced DNA damage in hemolymph cells with a time-response effect. Ionic forms presented higher genotoxicity than NPs, suggesting different mechanisms of action that may be mediated through oxidative stress.
Adam et al. (2015) demonstrated that nCuO had less negative effect than Cu salt on growth and reproduction of D. magna.
Fe NPs
Low toxicity and special surface chemistry of nFe2O3 widespread its use in biomedical applications like cellular labeling, drug delivery, tissue repair, in vitro bioseparation, and hyperthermia, with other applications like water and wastewater treatment (Chen et al. 2011), and in aquaculture as food supplement (Ren et al. 2011).
Chen et al. (2011) exposed medaka fish (Oryzias latipes) to nFe for 14 days evidencing lethal and sub-lethal effects (ROS generation and CAT alteration), showing that coated NPs with carboxymethyl cellulose were less toxic than uncoated ones.
Karthikeyeni et al. (2013) biosynthetized nFe2O3 and evidenced that after 96 h exposure to Oreochromis mossambicus, hematological (RBC, WBC, Hb, HCT) and biochemical parameters (SGOT, SGPT) significantly changed. Chen et al. (2013) found after 7 days exposure of O. latipes to nFe0 high mortality due to a combination of hypoxia and ROS production.
Zhu et al. (2012) have investigated the effects of nFe2O3 on the embryonic development of zebrafish resulting in embryos mortality, hatching delay, and malformation after 7 days exposure to ≥10 mg/L.
In Falugi et al. (2012), groups of 5–10 adults of Paracentrotus lividus of a similar size (50–60 mm) were forced to ingest of metal oxide NPs (SnO2, CeO2, and Fe3O4) (nominal concentrations 10−2 and 10−4 g/L). Results showed that after 1–2 days, none of the treated organisms at 10−2 g/L nFe survived. Iron bioaccumulation in digestive apparatus, severe reduction in the number of stained vesicles, as well as down-expression of hsp70 and GRP 78 were observed. The exposure of nFe3O4 to M. galloprovincialis (50 nm, polyethylene glycol capped, 0.370 mg/L) showed an accumulation in digestive gland after 8 h (>90%) remaning after 72-h depuration (>75%) (Hull et al. 2013).
La NPs
Lanthanides are widely used in industry, medicine (Mácová et al. 2014), and for water treatment (Rather et al. 2011). Mácová et al. (2014) exposed for 96 h juveniles of D. rerio and P. reticulate, and for 144 h embryonic stages of D. rerio, reporting the following LC50 values 156.33 ± 5.59 and 128.38 ± 5.29 mg/L, and 152.98 ± 8.06 mg/L, in that order. Thus, potential toxicity events could be associated to the use of La NPs.
Lürling and Tolman (2010) exposed D. magna for 14 days to different concentrations of La-QD, observing a size decrease in organisms after the first reproduction, but with no changes in the reproductive age and number of offspring.
Balusamy et al. (2015) exposed Chlorella sp. to up to 1000 mg/L of La-QD, and fed it to D. magna. Results evidenced that both Chlorella sp. biomass and D. magna mobility decreased. The LC50 value for La-QD for D. magna was 500 mg/L, and after 48 h at 1000 mg/L, the mortality of eposed daphins was 70%.
Quantum dots
QDs are used in electronic bioimaging, and biosensing (Feswick et al. 2013), and recently for water quality monitoring (Vázquez-González and Carrillo-Carrion 2014).
Louis et al. (2010) showed that O. mykiss exposed to 2 μg/L of QDs for 48 h presented an increase in total metallothioneins and LPO. Lewinski et al. (2011) exposed A. franciscana and D. magna for 24 h to 0.6 mg/L of QD. These microorganisms were fed to juvenile and adult of zebrafish for 21 days. Results showed no mortality after exposure, but QDs accumulated up to 4 and 8% for juveniles and adults, respectively. Gagné et al. (2008) obtained similar results after in vitro study with hepatocyte of O. mykiss.
Jackson et al. (2012) investigated the effects of QD-spiked algae (3.6 mg/L) fed to Leptocheirus plumulosus compared to water spiked with QDs. Results showed that mortality increased after 4 h exposure in a concentration-dependent manner in both administration routes with QD accumulation.
Kim et al. (2010) studied the influence of light wavelength on QD LC50 on D. magna evidencing after 48 h exposure. Toxicity increased from darkness to white fluorescence light, natural sunlight, and up to UV-B. Moreover, the QDs’ coatings seemed to be able to influence its toxicity, changing its stability and the potential release of toxic components (Kim et al. 2010; Feswick et al. 2013).
Selenium NPs
Se is an essential trace element required in diet for normal growth and physiological function of several organisms (Polettini et al. 2015), including fish (Khan et al. 2016); thus, it is an excellent bionutrient product for aquaculture enhancement. Khan et al. (2016) investigated the effects of dietary supplementation of nSe (0.68 mg/kg feed) on physiological and biochemical aspects of juvenile mahseer fish (Tor putitora), evidencing an increase in red blood cell count, hemoglobin level, hematocrit values, and lysozyme activity compared to the traditional diet as well as other biochemical parameters (serum growth hormone levels, tissue total protein content, and GSH-Px activity in liver and muscle tissues).
Silicon dioxide NPs
SiO2 NPs (nSiO2) are effective for drug delivery and optical imaging (Ramesh et al. 2013), but applications in aquaculture were reported as well in order to reduce the risk of disease spread in crowded fish pools (Strømme et al. 2009). Anyhow, Duan et al. (2013) observed an increase in zebrafish mortality and malformation after 96 h exposure to Si NPs.
Sn oxide NPs
Tin oxide NPs (nSnO2) present unique features such as rigid structure and low-temperature conductivity attracting great interest especially in the development of gas sensors, optoelectronic devices, catalysis, and electrochemical energy storage. Little data are available on nSnO2 toxicity on aquatic organisms, and its potential applications in aquaculture are still under evalution with information on only two species of potential interest. Krysanov et al. (2009) exposed P. reticulata to 150 mg/L of nSnO2 for 5 days, showing that tin accumulated in gill, spleen, intestine, liver, gonad, thymus, and muscle. Falugi et al. (2012) reporting P. lividus effects on nSnO2 were already discussed in the “Fe NPs” section.
SWCNTs
Carbon nanotubes (CNTs) present unique properties including high electrical conductivity, very high tensile strength, and hydrophobicity, which are valuable for wide-ranging industrial and biomedical applications such as electronic, drug delivery, and biosensing technology (McEuen et al. 2002; Galloway et al. 2010). In aquaculture, CNTs are used to increase food stability and promote water treatment (Fraser et al. 2011; Ren et al. 2011).
Fraser et al. (2011) compared the potential toxicity of SWCNT and C60. After 6 weeks feeding rainbow trouts (Oncorhynchus mykiss) by supplemented diet (500 mg SWCNT or C60), SWCNT had toxic effects, but C60 had not significanty effect on thiobarbituric acid reactive substances (TBARS—an indication of LPO) compared to the control. Smith et al. (2007) found after 10 days of exposure to SWCNT to O. mykiss damaged gill structures, and breathing and osmoregulation adversely affected, while TBARS decreased and total glutathione levels increased.
Petersen et al. (2008) investigated sediment samples spiked with SWCNTs and multiwalled carbon nanotubes (MWNTs) exposed to Lumbriculus variegatus, looking for uptake and depuration kinetics. Depuration behaviors suggested that nanotubes detected within the organisms were associated to the sediment remaining in organism guts, and not absorbed by tissues.
De Marchi et al. (2017) assessed the toxic effects of MWCNTs (0.01; 0.10 and 1.00 mg/L) in Diopatra neapolitana and Hediste diversicolor (regenerative capacity and respiration rate) and biochemical performance (energy reserves, metabolic activities, oxidative stress-related biomarkers, and neurotoxicity markers) after 28 days of exposure. They evidenced that exposure to MWCNTs induced negative effects on the regenerative capacity of D. neapolitana, stimulated its respiration rate (at higher concentrations), and altered energy-related responses (higher values of electron transport system activity, glycogen, and protein concentrations) In addition, both species showed oxidative stress with higher LPO, lower ratio between reduced and oxidized glutathione, and higher activity of antioxidant (CAT and SOD) and biotransformation (glutathione-S-transferases) enzymes in exposed organisms.
Titanium dioxide NPs
nTiO2 is used in several commercially available products such as paints, papers, textiles, plastics, sunscreens, cosmetics, and food products (Zhu et al. 2010a, b). As reviewed in the previous section, nTiO2 can be used both directly and indirectly in aquaculture (Sonawane et al. 2003; Ramsden et al. 2009). Therefore, it is necessary to investigate its potential toxicity in aquatic organisms.
Embryos of D. rerio were exposed during 96 h to different concentrations of nTiO2 in the form of anatase (TA) or anatase/rutile mixture (TM), under either visible light or a combination of visible and ultraviolet light (UV). Results showed that both cristallographic forms of nTiO2 caused accelerated hatching of larvae, alteration of the antioxidant enzymes (CAT and GSTs), and increased malformation of larvae (Clemente et al. 2014). nTiO2 facilitated the transport of Cd into carp (Cyprinus carpio) after 20 days of exposure (Zhang et al. 2007).
Bivalvia are highly vulnerable to ingestion of NPs from the water column. The NP uptake primarily occurs via the gills, and for this reason, it would be expected a higher accumulation in these tissues (Canesi et al. 2012). Mytilus galloprovincialis of 4–5 cm were kept for 24 h under static test condition containing different concentrations of nTiO2 (Canesi et al. 2010a, 2010b). Results showed that NPs induced significantly lysosomal membrane destabilization and lysosomal lipofuscin accumulation both in hemocytes and in digestive gland, as well as increase GSTs in gills. Abalones (Haliotis diversicolor) were exposed to lethal concentrations of nTiO2 and after 96 h exposure showing an increase of the lipid peroxidation (LPO), and a decrease in GSH activity and nitric oxide production (Zhu et al. 2010a, b).
Artemia spp., Daphnia spp., Ceriodaphnia dubia, and Lumbrinereis variegates are used as live food source in freshwater larviculture, and in ecotoxicological studies (García-Alonso et al. 2011; Jackson et al. 2012; Feswick et al. 2013). A. salina is one of the most studied organisms in marine ecotoxicity (Radhika Rajasree et al. 2010; Libralato et al. 2016b). In Ates et al. (2013), A. salina was exposed to different concentrations of nTiO2. Their results showed that after 96 h exposure, no mortality occured and LPO levels did not change (Libralato 2014).
Acute and chronic ecotoxicity nTiO2 studies on D. magna showed a dose-dependent mortality (Zhu et al. 2010a, b). Results showed that Ti accumulated in the gut, but did not cause any immobilization (Amiano et al. 2012). Lovern et al. (2007) reported concentration-dependent mortality of D. magna exposed to filtered nTiO2 (≈30 nm), with an LC50 5.5 mg/L. An EC50 >100 mg/L was reported by Warheit et al. (2007) and Zhu et al. (2010a, b) for nTiO2 (100–140 nm) for D. magna after 48 h of exposure. Wiench et al. (2009) found EC50 >100 mg/L for both uncoated and coated TiO2 NPs. Amiano et al. (2012) found an EC50 = 3.4 mg/L of nTiO2 after exposure to 0.56 mW/cm2 UVA radiation using river water as testing matrix. Marcone et al. (2012) showed no toxicity of TiO2 during light and dark conditions up to 100 mg/L. Dalai et al. (2013) evidenced after 48 h two EC50s considering light (8.26 mg/L) and dark (33.65 mg/L) scenarios fo C. dubia.
A synergistic effect of nTiO2 and As5+ was observed on C. dubia showing that at low concentrations of nTiO2, the toxicity of As5+ can significantly increase (Wang et al. 2011). Arenicola marina was exposed for 10 days to SWNT (0.003–0.03 g/kg) and nTiO2 (1–3 g/kg) (Galloway et al. 2010). Results showed that SWCNTs did not affect feeding behavior, but nTiO2 did, in addition to lysosomal stability causing DNA damage.
Zinc oxide NPs
ZnO NPs are used in optoelectronics, cosmetics, catalysts, ceramics, pigments (Bai et al. 2010), and aquaculture (Faiz et al. 2015). Contradictory results exist about nZnO effects according to concetrations, contact time, and target organisms (Berube 2008).
Bai et al. (2010) exposed zebrafish embryos to various concentrations of nZnO, showing a significant decrease in survival, hatching, and larval growth rate after 94 h. Hao et al. (2013) carried out a 30-day study on juvenile of C. carpio exposed to nZnO, highlighting severe histopathological alterations and intracellular oxidative stress. Muralisankar et al. (2014) demostrated that M. rosenbergii after 90 days exposure to nZnO showed impaired growth and survival rates, and alterations in the activities of digestive enzymes (protease, amylase, and lipase), and biochemical constituents (total protein, total amino acid, total carbohydrate, and total lipid).
Trevisan et al. (2014) showed that after 96 h exposure of Crassostrea gigas to lethal concentration of nZnO (30 mg/L), Zn accumulated in gill and in digestive glands, causing oxidative damage. O’Rourke (2013) evidenced that short-term exposure (up to 96 h) to lethal concentrations of nZnO (10 mg/L) had no negative effect on Lumbriculus variegatus, but long exposure (up to 28 days) showed toxic effects.
The effects of nZnO evidenced effects on embryo development, Zn bioaccumulation, oxidative stress, and behavior according to exposure scenario and target organisms. In aquaculture, the use of nZnO can improve growth and immune response and the quality of water in fishponds, but waterborne and dietary exposure can have also undesired toxic effects. Focused studies are required to determine the safe exposure concentration of nZnO for aquaculture activity.
Conclusions
Nanotechnology is still in its infancy in aquaculture with just few applications documented mainly in the packaging sector. Little data were produced with the specific aim of checking the effects of NPs on aquacultered species, while several NPs are used in aquaculture. Thus, aquaculture must pay great attention in keeping food security along the production process in a cradle-to-grave perspective considering both human health and the environment potential adverse effects of NPs. Currently, it is unworthy to provide a toxicity ranking of NPs in aquaculture, mainly because the amount of information is aggregated just on few NPs (nAg and nTiO2) with scattered data for all the remainings. Thus, results could be strongly unbalanced.
Researchers and manufacturers are trying to use nano-based tools to remove the barriers about waterborne food, growth, reproduction, and culturing of species, their health, and water treatment in order to increase aquaculture production rates. Anyway, nanosafety-related concerns still exist and must be tackled before their full-scale implementation. Toxicological effects of NPs depend on various factors including complex interplay between particle features (e.g., diameter, form, surface charge, and chemistry), concentration, time of exposure, nature of the NPs, medium composition, route of particle administration, and target species immune system. Despite the available information, several points of criticism are hindering the exact understanding of NPs safety in aquaculture. Firstly, the way NPs are used in aquaculture can be very different: addition to food, to water media, or in aquaculture facilities (i.e., surface treatments). Nevertheless, the existing amount of studies in aquatic toxicology, the available exposure scenarios, are inadequate to fullfill the request for NP safety in aquaculture like as their route of administration, their concentration, and exposure time. Concentrations are sometimes lower (i.e., concentration administered via feed) or higher (i.e., concentration administered via water or surface treatments) than what it is applied or expected to be applied in aquaculture leading to unrealistic results. Thus, it is not possibile to infer about the potential adverse effects on the final consumer. It is necessary to explore the safety of nano-based aquaculture considering not only relatively short-term treatment periods (<40 days) but also the whole aquacultured products along their life cycle from the egg/larva to the table, including water quality. Moreover, due to the fact that aquatic organisms are cultured in different environments (e.g., freshwater and saltwater or tropical and temperate regions), nano-based products can behave very differently like as the derived effects; thus, it would be interesting to explore how nanosafety could be influenced by environmental factors mainly salinity, pH, and temperature.
Abbreviations
- NPs:
-
Nanoparticles
- nTiO2 :
-
Titanium dioxide NPs
- selenium:
-
NPs nSe
- nZnO:
-
Zinc oxide NPs
- nFe:
-
Iron NPs
- nSiO2 :
-
Silicon dioxide NPs
- nAu:
-
Gold NPs
- SWCNTs:
-
Single-walled carbon nanotubes
- C60 :
-
Fullerene
- nAg:
-
Silver NPs
- QDs:
-
Quantum dots
- nSnO2 :
-
Tin oxide NPs
- nCeO2 :
-
Cerium oxide NPs
- nAl2O3 :
-
Aluminum oxide NPs
- nCuO:
-
Copper oxide NPs
- ZFL:
-
Zebrafish liver cell line
- WSSV:
-
White spot syndrome virus
- CAgNCs:
-
Chitosan-silver nano composites
- MIC:
-
Minimum inhibitory concentration
- RFID:
-
Radio frequency identification
- DAG-PEG:
-
Diacylglycerol-polyethyleneglycol
- Cu2+-MMT:
-
Copper-bearing nanomontmorillonite
- TBT:
-
Antifouling pesticide tributyltin
- HSP:
-
Heat shock proteins
- CYP:
-
Cytochrome P450
- HEK 293:
-
Human embryonic kidney 293 cells
- UV:
-
Ultraviolet light
- EC50 :
-
Half maximal effective concentration
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- LPO:
-
Lactoperoxidase
- GOT:
-
Glutamic-oxaloacetic transaminase
- GPT:
-
Glutamic-pyruvic transaminase
- GSTs:
-
Glutathione-S-transferase
- ROS:
-
Reactive oxygen species
- RBC:
-
Red blood cell
- WBC:
-
White blood cell
- HB:
-
Hemoglobin
- HTC:
-
Hematocrit
- SGOT:
-
Serum glutamic-oxaloacetic transaminase
- SGPT:
-
Serum glutamic-pyruvic transaminase
- GRP:
-
Glucose-regulated protein
- LMS:
-
Lysosomal membrane stability
- TBARS:
-
Thiobarbituric acid reactive substances
- MWNTs:
-
Multiwalled carbon nanotubes
- PVP:
-
Polyvinylpyrrolidone
- PEG:
-
Polyethylene glycol
- LC50 :
-
Lethal concentration 50
- BHAL:
-
Bi-potential human liver cells
- GSH-Px:
-
Glutathione peroxidase
References
Adam N, Vakurov A, Knapen D, Blust R (2015) The chronic toxicity of CuO nanoparticles and copper salt to Daphnia magna. J Hazard Mater 283:416–422
Aitken R, Chaudhry M, Boxall A, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup med 56:300–306
Alishahi A, Proulx J, Aider M (2014) Chitosan as biobased nanocomposite in seafood industry and aquaculture seafood science. Ad Chem Technol Appl 211
Amiano I, Olabarrieta J, Vitorica J, Zorita S (2012) Acute toxicity of nanosized TiO2 to Daphnia magna under UVA irradiation. Environ Toxicol Chem 31(11):2564–2566
Arulvasu C, Jennifer SM, Prabhu D, Chandhirasekar D (2014) Toxicity effect of silver nanoparticles in brine shrimp Artemia. Sci World J 2014
Asche F, Bellemare MF, Roheim C, Smith MD, Tveteras S (2015) Fair enough? Food security and the international trade of seafood. World dev 31(67):151–160
Asharani P, Lianwu Y, Gong Z, Valiyaveettil S (2011) Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicol 5:43–54
Ashraf PM, Edwin L (2016) Nano copper oxide incorporated polyethylene glycol hydrogel: an efficient antifouling coating for cage fishing net. Int Biodeter Biodegr 115:39–48
Ates M, Daniels J, Arslan Z, Farah IO (2013) Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess 185:3339–3348
Bai W, Zhang Z, Tian W, He X, Ma Y, Zhao Y, Chai Z (2010) Toxicity of zinc oxide nanoparticles to zebrafish embryo: a physicochemical study of toxicity mechanism. J Nanopart res 12:1645–1654
Balusamy B, Taştan BE, Ergen SF, Uyar T, Tekinay T (2015) Toxicity of lanthanum oxide (La2O3) nanoparticles in aquatic environments. Environ Sci: Processes & Impacts 17:1265–1270
Barber D, Garcia N, Denslow N, Hyndman K, Evans D, Freedman J (2005) Effects of aluminum nanoparticle exposure in zebrafish (Danio rerio) SETAC
Barelds HP (2010) The uptake and effects on survival of nano silver and nano titanium dioxide in brine shrimp (Artemia nauplii). PhD thesis. Faculty of Veterinary Medicine Theses, University of Utrecht, Netherlands. Available from: http://dspace.library.uu.nl/handle/1874/44547
Berube DM (2008) Rhetorical gamesmanship in the nano debates over sunscreens and nanoparticles. J Nanopart res 10:23–37
Bhat IA, Rather MA, Saha R, Pathakota G-B, Pavan-Kumar A, Sharma R (2016) Expression analysis of Sox9 genes during annual reproductive cycles in gonads and after nanodelivery of LHRH in Clarias batrachus. Res vet Sci 106:100–106. doi:10.1016/j.rvsc.2016.03.022
Bhattacharyya A, Reddy SJ, Hasan MM, Adeyemi M, Marye RR (2015) Nanotechnology-a unique future technology in aquaculture for the food security. Int J Bioass 4:4115–4126
Bilberg K, Malte H, Wang T, Baatrup E (2010) Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis). Aquat Toxicol 96(2):159–165
Bisesi JH et al (2015) Examination of single-walled carbon nanotubes uptake and toxicity from dietary exposure: tracking movement and impacts in the gastrointestinal system. Nano 5:1066–1086
Blinova, I., Niskanen, J., Kajankari, P., Kanarbik, L., Käkinen, A., Tenhu, H., ... & Kahru, A. (2013). Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environmental Science and Pollution Research, 20(5), 3456–3463.
Buffet P-E et al (2011) Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere 84:166–174
Callegaro S, Minetto D, Pojana G, Bilanicová D, Libralato G, Volpi Ghirardini A, Hassellöv M, Marcomini A (2015) Effects of alginate on stability and ecotoxicity of nano-TiO2 in artificial seawater. Ecotoxicol Environ Saf 117:107–114
Canesi L, Ciacci C, Vallotto D, Gallo G, Marcomini A, Pojana G (2010a) In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat Toxicol 96:151–158
Canesi L, Fabbri R, Gallo G, Vallotto D, Marcomini A, Pojana G (2010b) Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nano-particles (nano carbon black, C60 fullerene, nano-TiO2, nano-SiO2). Aquat Toxicol 100:168e177
Canesi L, Ciacci C, Fabbri R, Marcomini A, Pojana G, Gallo G (2012) Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar Environ res 76:16–21
Castillo-Rodal A, Mazari-Hiriart M, Lloret-Sánchez L, Sachman-Ruiz B, Vinuesa P, López-Vidal Y (2012) Potentially pathogenic nontuberculous mycobacteria found in aquatic systems. Analysis from a reclaimed water and water distribution system in Mexico City. Eur J Clin Microbiol Infec dis 31:683–694
Chalamcheria V (2015) Nano vaccines: new paradigm in aqua health sector. J Aquac mar Biol 3(2):00061
Champ MA (2003) Economic and environmental impacts on ports and harbors from the convention to ban harmful marine anti-fouling systems. Mar Pollut Bull 46:935–940
Chatterjee S, Judeh ZM (2016) Impact of encapsulation on the physicochemical properties and gastrointestinal stability of fish oil. LWT-Food Sci Techn 65:206–213
Chen P-J, Su C-H, Tseng C-Y, Tan S-W, Cheng C-H (2011) Toxicity assessments of nanoscale zerovalent iron and its oxidation products in medaka (Oryzias latipes) fish. Mar Pollut Bull 63:339–346
Chen P-J, Wu W-L, Wu KC-W (2013) The zerovalent iron nanoparticle causes higher developmental toxicity than its oxidation products in early life stages of medaka fish. Water res 47:3899–3909
Clemente Z, Castro V, Moura M, Jonsson C, Fraceto L (2014) Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat Toxicol 147:129–139
Cong Y, Banta GT, Selck H, Berhanu D, Valsami-Jones E, Forbes VE (2011) Toxic effects and bioaccumulation of nano-, micron- and ionic-Ag in the polychaete, Nereis diversicolor. Aquat Toxicol 105:403–411
Cozzari M, Elia AC, Pacini N, Smith BD, Boyle D, Rainbow PS, Khan FR (2015) Bioaccumulation and oxidative stress responses measured in the estuarine ragworm (Nereis diversicolor) exposed to dissolved, nano-and bulk-sized silver. Environ Pollut 198:32–40
Dalai S, Pakrashi S, Chandrasekaran N, Mukherjee A (2013) Acute toxicity of TiO 2 nanoparticles to Ceriodaphnia dubia under visible light and dark conditions in a freshwater system. PLoS One 8(4):e62970
Dananjaya S, Godahewa G, Jayasooriya R, Lee J, De Zoysa M (2016) Antimicrobial effects of chitosan silver nano composites (CAgNCs) on fish pathogenic Aliivibrio (Vibrio) salmonicida. Aquaculture 450:422–430
Dasgupta N, Ranjan S, Mundekkad D, Ramalingam C, Shanker R, Kumar A (2015) Nanotechnology in agro-food: from field to plate. Food res Int 69:381–400
De Campos AM, Diebold Y, Carvalho EL, Sánchez A, Alonso MJ (2004) Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm res 21:803–810
De Marchi L, Neto V, Pretti C, Figueira E, Chiellini F, Soares AMVM, Freitas R (2017) Physiological and biochemical responses of two keystone polychaete species: Diopatra neapolitana and Hediste diversicolor to multi-walled carbon nanotubes. Environ res 154:126–138
Deng Y, Cheng Q (2003) Affects of nano-seleniumon the growth of nile tilapia (Oreochromis niloticus). Inland Aquat Prodd 6:28–30
Dodane V, Khan MA, Merwin JR (1999) Effect of chitosan on epithelial permeability and structure. Int J Pharm 182:21–32
Duan J, Yu Y, Li Y, Yu Y, Sun Z (2013) Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials 34(23):5853–5862
ETC (Action Group on Erosion, Technology and Concentration) (2003) Down on the farm: the impact of nanoscale technologies on food and agriculture. [http://www.etcgroup.org/en/materials/publications.html?pub_id=80]
Faiz H, Zuberi A, Nazir S, Rauf M, Younus N (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int J Agr Biol 17:568–574
Falugi C et al (2012) Toxicity of metal oxide nanoparticles in immune cells of the sea urchin. Mar Environ res 76:114–121
Ferosekhan S; Gupta S; Singh AR; AM Rather; R Kumari; DC Kothari; AK Pal; SB Jadhao 2014. RNA-loaded chitosan nanoparticles for enhanced growth, immunostimulation and disease resistance in fish. Curr Nanosci 10(3), 453–464.
Feswick A, Griffitt RJ, Siebein K, Barber D (2013) Uptake, retention and internalization of quantum dots in Daphnia is influenced by particle surface functionalization. Aquat Toxicol 130:210–218
Fotedar R, Munilkumar S (2016) Effects of organic selenium supplementation on growth, glutathione peroxidase activity and histopathology in juvenile barramundi (Lates calcarifer Bloch 1970) fed high lupin meal-based diets. Aquaculture 457:15–23
Fraser TW, Reinardy HC, Shaw BJ, Henry TB, Handy RD (2011) Dietary toxicity of single-walled carbon nanotubes and fullerenes (C60) in rainbow trout (Oncorhynchus mykiss). Nanotoxicol 5:98–108
Gagné F, Maysinger D, André C, Blaise C (2008) Cytotoxicity of aged cadmium-telluride quantum dots to rainbow trout hepatocytes. Nanotoxicol 2:113–120
Galloway T, Lewis C, Dolciotti I, Johnston BD, Moger J, Regoli F (2010) Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ Pollut 158:1748–1755
García-Alonso J et al (2011) Cellular internalization of silver nanoparticles in gut epithelia of the estuarine polychaete Nereis diversicolor. Environ Sci Technol 45:4630–4636
García-Negrete CA, Blasco J, Volland M, Rojas TC, Hampel M, Lapresta-Fernández A et al (2013) Behaviour of Au-citrate nanoparticles in seawater and accumulation in bivalves at environmentally relevant concentrations. Environ Pollut 174:134–141
García-Rodríguez D, Carro AM, Lorenzo RA, Fernández F, Cela R (2008) Determination of trace levels of aquaculture chemotherapeutants in seawater samples by SPME-GC-MS/MS. J Sep Sci 31:2882–2890
George M, Abraham TE (2006) Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release 114:1–14
Gerber LC, Moser N, Luechinger NA, Stark WJ, Grass RN (2012) Phosphate starvation as an antimicrobial strategy: the controllable toxicity of lanthanum oxide nanoparticles. Chem Commun 48:3869–3871
Gomes T, Araújo O, Pereira R, Almeida AC, Cravo A, Bebianno MJ (2013) Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar Environ res 84:51–59
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Guo H, Lai Q, Wang W, Wu Y, Zhang C, Liu Y, Yuan Z (2013) Functional alginate nanoparticles for efficient intracellular release of doxorubicin and hepatoma carcinoma cell targeting therapy. Int J Pharm 451:1–11
Guo H, Hong Z, Yi R (2015) Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J Food Sci 80:N1595–N1601
Guo J, Zhang R, Yang N (2016) An Immunomagnetic-nanoparticle-based microfluidic system for rapid separation and detection of aquaculture pathogens. J Comput Theor Nanosci 13:2226–2231
Hao L, Chen L, Hao J, Zhong N (2013) Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): a comparative study with its bulk counterparts. Ecotoxicol Environ Saf 91:52–60
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2016) Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem 194:1266–1274
Hu Y-L, Qi W, Han F, Shao J-Z, Gao J-Q (2011) Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int J Nanomedicine 6:3351–3359
Hu J, Wang D, Wang J, Wang J (2012) Bioaccumulation of Fe2O3 (magnetic) nanoparticles in Ceriodaphnia dubia. Environ Pollut 162:216–222
Huang M, Khor E, Lim L-Y (2004) Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm res 21:344–353
Huang C-M, Chen C-H, Pornpattananangkul D, Zhang L, Chan M, Hsieh M-F, Zhang L (2011) Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 32:214–221
Huang S, Wang L, Liu L, Hou Y, Li L (2015) Nanotechnology in agriculture, livestock, and aquaculture in China. Rev Agron Sustain Dev 35:369–400
Hull MS, Vikesland PJ, Schultz IR (2013) Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis). Aquat Toxicol 140:89–97
Jackson BP, Bugge D, Ranville JF, Chen CY (2012) Bioavailability, toxicity, and bioaccumulation of quantum dot nanoparticles to the amphipod Leptocheirus plumulosus. Environ Sci Technol 46:5550–5556
Jang MH, Kim WK, Lee SK, Henry TB, Park JW (2014) Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environ Sci Technol 48(19):11568–11574
Ji J, Torrealba D, Ruyra À, Roher N (2015) Nanodelivery systems as new tools for immunostimulant or vaccine administration: targeting the fish immune system. Biology 4:664–696
Jiménez-Fernández E, Ruyra A, Roher N, Zuasti E, Infante C, Fernández-Díaz C (2014) Nanoparticles as a novel delivery system for vitamin C administration in aquaculture. Aquaculture 432:426–433
Johari SA, Kalbassi MR, Soltani M, Yu IJ (2015) Study of fungicidal properties of colloidal silver nanoparticles (AgNPs) on trout egg pathogen, Saprolegnia sp. Int J Aquat Biol 3:191–198
Johnston BD et al (2010) Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Environ Sci Technol 44:1144–1151
Kandasamy K, Alikunhi NM, Manickaswami G, Nabikhan A, Ayyavu G (2013) Synthesis of silver nanoparticles by coastal plant Prosopis chilensis (L.) and their efficacy in controlling vibriosis in shrimp Penaeus monodon. Appl Nanosci 3:65–73
Karnik BS, Davies SH, Baumann MJ, Masten SJ (2005) Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ Sci Technol 39:7656–7661
Karthikeyeni S, Siva Vijayakumar T, Vasanth S, Arul Ganesh MM, Subramanian P (2013) Biosynthesis of iron oxide nanoparticles and its haematological effects on fresh water fish Oreochromis mossambicus. J Acad Indus res 10:645–649
Katuli KK, Amiri BM, Massarsky A, Yelghi S (2014a) Impact of a short-term diazinon exposure on the osmoregulation potentiality of Caspian roach (Rutilus rutilus) fingerlings. Chemosphere 31(108):396–404
Katuli KK, Massarsky A, Hadadi A, Pourmehran Z (2014b) Silver nanoparticles inhibit the gill Na+/K+-ATPase and erythrocyte AChE activities and induce the stress response in adult zebrafish (Danio rerio). Ecotoxicol Environ Saf 106:173–180
Khan FR, Paul KB, Dybowska AD, Valsami-Jones E, Lead JR, Stone V, Fernandes TF (2015) Accumulation dynamics and acute toxicity of silver nanoparticles to Daphnia magna and Lumbriculus variegatus: implications for metal modeling approaches. Environ Sci Technol 49:4389–4397
Khan KU, Zuberi A, Nazir S, Fernandes JBK, Jamil Z, Sarwar H (2016) Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk J Zool 40
Kim J, Park Y, Yoon TH, Yoon CS, Choi K (2010) Phototoxicity of CdSe/ZnSe quantum dots with surface coatings of 3-mercaptopropionic acid or tri-n-octylphosphine oxide/gum arabic in Daphnia magna under environmentally relevant UV-B light. Aquat Toxicol 97:116–124
Klinkesorn U, McClements DJ (2009) Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem 114:1308–1315
Krishnaraj C, Harper SL, Yun SI (2016) In vivo toxicological assessment of biologically synthesized silver nanoparticles in adult zebrafish (Danio rerio). J Hazard Mater 301:480–491
Krysanov EY, Demidova TB, Pel’gunova LA, Badalyan SM, Rumyantseva MN, Gas’ kov AM (2009) Effect of hydrated tin dioxide (SnO2· xH2O) nanoparticles on guppy (Poecilia reticulata Peters, 1860). In: Doklady Biological Sciences, vol 426, no. 1. MAIK Nauka/Interperiodica, USA, pp. 288-289.
Lara HH, Ayala-Núnez NV, Turrent LCI, Padilla CR (2010) Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 26:615–621
Lewinski NA, Zhu H, Ouyang CR, Conner GP, Wagner DS, Colvin VL, Drezek RA (2011) Trophic transfer of amphiphilic polymer coated CdSe/ZnS quantum dots to Danio rerio. Nano 3:3080–3083
Libralato G (2014) The case of Artemia spp. in nanoecotoxicology. Mar Environ res 101:38–43
Libralato G et al (2013) Embryotoxicity of TiO2 nanoparticles to Mytilus galloprovincialis (Lmk). Mar Environ res 92:71–78
Libralato G et al (2016a) Phytotoxicity of ionic, micro-and nano-sized iron in three plant species. Ecotoxicol Environ Saf 123:81–88
Libralato G, Prato E, Migliore L, Cicero A, Manfra L (2016b) A review of toxicity testing protocols and endpoints with Artemia spp. Ecol Indic 69:35–49
Liu P-W, Guo T, Wei H (2009) Bactericidal effects of copper-bearing nano-montmorillonite on three aquatic pathogenic bacteria and two intestinal available bacteria in vitro. J Shanghai Ocean Univ 5:002
Lofrano G et al (2016a) Polymer functionalized nanocomposites for metals removal from water and wastewater: an overview. Water res 92:22–37
Lofrano G, Libralato G, Alfieri A, Carotenuto M (2016b) Metals and tributyltin sediment contamination along the Southeastern Tyrrhenian Sea coast. Chemosphere 144:399–407. doi:10.1016/j.chemosphere.2015.09.002
Loh JW, Yeoh G, Saunders M, Lim L-Y (2010) Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol Appl Pharmcol 249:148–157
Louis S, Gagné F, Auclair J, Turcotte P, Gagnon C, Émond C (2010) The characterisation of the behaviour and gill toxicity of CdS/CdTe quantum dots in rainbow trout (Oncorhynchus mykiss). Int J Biomed Nanosci Nanotechnol 1:52–69
Lovern SB, Strickler JR, Klaper R (2007) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol 41:4465–4470
Luo Y, Wang Q (2013) Recent advances of chitosan and its derivatives for novel applications in food science. J Food Process Beverag 1:1–13
Lürling M, Tolman Y (2010) Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna. Water res 44:309–319
Mácová S, Babula P, Plhalová L, Žáčková K, Kizek R, Svobodová Z (2014) Acute toxicity of lanthanum to fish Danio rerio and Poecilia reticulata. J Biochem Technol 2:46–47
Marcone GP, Oliveira ÁC, Almeida G, Umbuzeiro GA, Jardim WF (2012) Ecotoxicity of TiO2 to Daphnia similis under irradiation. J Hazard Mater 211:436–442
McEuen PL, Fuhrer MS, Park H (2002) Single-walled carbon nanotube electronics. IEEE Trans Nanotechnol 1:78–85
Minetto D, Libralato G, Volpi Ghirardini A (2014) Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: an overview. Environ Int 66(0):18–27
Minetto D, Volpi Ghirardini A, Libralato G, (2016) Saltwater ecotoxicology of nAg, nAu, nCuO, nTiO2, nZnO and C60. Environ Int
Mohd Ashraf M, Aklakur R, Shabir A, Mujhid K (2011) Nanotechnology as a novel tool in fisheries and aquaculture development: a review. Iran J Energy Environ 2(3):258–261
Mouneyrac C et al (2014) Fate and effects of metal-based nanoparticles in two marine invertebrates, the bivalve mollusc Scrobicularia plana and the annelid polychaete Hediste diversicolor. Environ Sci Pollut res 21:7899–7912
Mühling M, Bradford A, Readman JW, Somerfield PJ, Handy RD (2009) An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar Environ res 68:278–283
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Manickam N, Srinivasan V (2014) Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol Trace Elem res 160:56–66
O’Rourke SA (2013) The environmental toxicology of zinc oxide nanoparticles to the oligochaete. Heriot-Watt University
Osborne OJ, Lin S, Chang CH, Ji Z, Yu X, Wang X, Lin S, Xia T, Nel AE (2015) Organ-specific and size-dependent Ag nanoparticle toxicity in gills and intestines of adult zebrafish. ACS nano 9(10):9573–9584
Pace HE, Lesher EK, Ranville JF (2010) Influence of stability on the acute toxicity of CdSe/ZnS nanocrystals to Daphnia magna. Environ Toxicol Chem 29:1338–1344
Pati R, Mehta RK, Mohanty S, Padhi A, Sengupta M, Vaseeharan B, Goswami C, Sonawane A (2014) Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomedicine: NBM 10:1195-1208
Peniche C, Howland I, Carrillo O, Zaldıvar C, Argüelles-Monal W (2004) Formation and stability of shark liver oil loaded chitosan/calcium alginate capsules. Food Hydrocoll 18:865–871
Perera TS, Han Y, Lu X, Wang X, Dai H, Li S (2015) Rare earth doped apatite nanomaterials for biological application. J Nanomat 1(2015):5
Petersen EJ, Huang Q, Weber WJ Jr (2008) Ecological uptake and depuration of carbon nanotubes by Lumbriculus variegatus. Environ Health Perspect 116:496
Podyacheva OY, Ismagilov ZR (2015) Nitrogen-doped carbon nanomaterials: to the mechanism of growth, electrical conductivity and application in catalysis. Catal Today 1(249):12–22
Polettini A-E, Fortaner S, Farina M, Groppi F, Manenti S, Libralato G, Sabbioni E (2015) Uptake from water, internal distribution and bioaccumulation of selenium in Scenedesmus obliquus, Unio mancus and Rattus norvegicus: part A. Bull Environ Contam Toxicol 94(1):84–89
Pradeep T (2009) Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517:6441–6478
Pulavendran S, Chellan R, Asit B (2011) Hepatocyte growth factor incorporated chitosan nanoparticles augment the differentiation of stem cell into hepatocytes for the recovery of liver cirrhosis in mice. J Nanobiotechnol 9(1):1
Pulkkinen K et al (2010) Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc R Soc Lond B Biol Sci 277. 1681:593–600
Radhika Rajasree S, Ganesh Vumar V, Stanley Abraham L, Indabakandan D (2010) Studies on the toxicological effects of engineered nanoparticles in environment—a review. Int J Appl Bioeng 4
Rafiee A et al (2014) Comparison of chitosan, alginate and chitosan/alginate nanoparticles with respect to their size, stability, toxicity and transfection. Asian Pac J Trop dis 4:372–377
Rajeshkumar S, Venkatesan C, Sarathi M, Sarathbabu V, Thomas J, Basha KA, Hameed AS (2009) Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish & Shellfish Immunol 26:429–437
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6(1):19–29
Ramesh R, Kavitha P, Kanipandian N, Arun S, Thirumurugan R, Subramanian P (2013) Alteration of antioxidant enzymes and impairment of DNA in the SiO2 nanoparticles exposed zebra fish (Danio rerio). Environ Monit Assess 185:5873–5881
Ramezani Z, Zarei M, Raminnejad N (2015) Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control 51:43–48
Ramsden CS, Smith TJ, Shaw BJ, Handy RD (2009) Dietary exposure to titanium dioxide nanoparticles in rainbow trout (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology 18:939–951
Ramya VL, Sharma R, Gireesh-Babu P, Patchala SR, Rather A, Nandanpawar PC, Eswaran S (2014) Development of chitosan conjugated DNA vaccine against nodavirus in Macrobrachium rosenbergii (De Man, 1879). J Fish dis 37:815–824. doi:10.1111/jfd.12179
Rather M, Sharma R, Aklakur M, Ahmad S, Kumar N, Khan M, Ramya V (2011) Nanotechnology: a novel tool for aquaculture and fisheries development. A prospective mini-review. Fisheries Aquaculture J 16:1–5
Rather MA, Sharma R, Gupta S, Ferosekhan S, Ramya V, Jadhao SB (2013) Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS One 8:e57094
Rather MA, Bhat IA, Gireesh-Babu P, Chaudhari A, Sundaray JK, Sharma R (2016) Molecular characterization of kisspeptin gene and effect of nano-encapsulted kisspeptin-10 on reproductive maturation in Catla catla. Domest Anim Endocrinol 56:36–47. doi:10.1016/j.domaniend.2016.01.005
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170:395–410
Romero J, Feijoó, CG, Navarrete P (2012) Antibiotics in Aquaculture – Use, Abuse and Alternatives, Health and Environment in Aquaculture. In: Carvalho E (ed), InTech. doi:10.5772/28157. Available from: https://www.intechopen.com/books/health-and-environment-in-aquaculture/antibiotics-in-aquaculture-use-abuse-and-alternatives
Ross KA, Loyd H, Wu W, Huntimer L, Wannemuehler MJ, Carpenter S, Narasimhan B (2014) Structural and antigenic stability of H5N1 hemagglutinin trimer upon release from polyanhydride nanoparticles. J Biomed mat ResPart a 102(11):4161–4168
Rotruck J, Pope A, Ganther H, Swanson A, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sabbioni E, Polettini A-E, Fortaner S, Farina M, Groppi F, Manenti S, Libralato G (2015) Uptake from water, internal distribution and bioaccumulation of selenium in Scenedesmus obliquus, Unio mancus and Rattus norvegicus: part B. Bull Environ Contam Toxicol 94(1):90–95
Shaalan M, Saleh M, El-Mahdy M, El-Matbouli M (2016) Recent progress in applications of nanoparticles in fish medicine: a review. Nanomedicine: NBM 12:701-710
Sharma N, Rather MA, Ajima MNO, Gireesh-Babu P, Kumar K, Sharma R (2016) Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nanoparticles. Food Chem Toxicol 96:122–132. doi:10.1016/j.fct.2016.06.020
Shirazi MA, Shariati F, Ramezanpour Z (2015) Toxic effect of aluminum oxide nanoparticles on green micro-algae dunaliella salina. Int J Environ Res 9(2): 585–594
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes to rainbow trout (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:94–109
Sonawane R, Hegde S, Dongare M (2003) Preparation of titanium (IV) oxide thin film photocatalyst by sol–gel dip coating. Mater Chem Phy 77:744–750
Sonkusre P, Nanduri R, Gupta P, Cameotra SS (2014) Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J Nanomed Nanotechnol
Strømme M, Brohede U, Atluri R, Garcia-Bennett AE (2009) Mesoporous silica-based nanomaterials for drug delivery: evaluation of structural properties associated with release rateWiley Interdisciplinary Reviews. Nanomed Nanobiotechnol 1:140–148
Swain P et al (2014) Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J Microbiol Biotechnol 30:2491–2502
Tedesco S, Doyle H, Redmond G, Sheehan D (2008) Gold nanoparticles and oxidative stress in Mytilus edulis. Mar Environ res 66:131–133
Trevisan R et al (2014) Gills are an initial target of zinc oxide nanoparticles in oysters Crassostrea gigas, leading to mitochondrial disruption and oxidative stress. Aquat Toxicol 153:27–38
Twiddy D, Reilly P, Chatham K (1995) Occurrence of antibiotic-resistant human-pathogens in integrated fish farms. FAO Fish rep 514:23
Vale G, Mehennaoui K, Cambier S, Libralato G, Jomini S, Domingos RF (2016) Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: a critical overview. Aquat Toxicol 170:162–174
Vázquez-González M, Carrillo-Carrion C (2014) Analytical strategies based on quantum dots for heavy metal ions detection. J Biomed opt 19:101503–101503
Vendramini T et al (2016) Effects of a blend of essential oils, chitosan or monensin on nutrient intake and digestibility of lactating dairy cows. Animal Feed Sci Technol 214:12–21
Venkat K (2011) The climate change and economic impacts of food waste in the United States. Int J Food Syst Dyn 2:431–446
Vijayan SR, Santhiyagu P, Singamuthu M, Kumari Ahila N, Jayaraman R, Ethiraj K (2014) Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci World J
Völker C, Kämpken I, Boedicker C, Oehlmann J, Oetken M (2015) Toxicity of silver nanoparticles and ionic silver: comparison of adverse effects and potential toxicity mechanisms in the freshwater clam Sphaerium corneum. Nanotoxicology 9(6):677–685
Wang H, Wick RL, Xing B (2009) Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut 157:1171–1177
Wang D, Hu J, Irons DR, Wang J (2011) Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia. Sci Total Environ 409:1351–1356
Wang Y, Yan X, Fu L (2013) Effect of selenium nanoparticles with different sizes in primary cultured intestinal epithelial cells of crucian carp, Carassius auratus gibelio. Int J Nanomedicine 8:4007–4013
Warheit DB, Hoke RA, Finlay C, Donne EM, Reed KL et al (2007) Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett 171:99–110
Wen J, Chai D, Ding Y, Yu L, Huang J (2003) Summary report on experiment of strong nanometer 863 biological assistant growth unit in sea shrimp farming. Modern Fisheries Inf 18:12–15
Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, Landsiedel R (2009) Acute and chronic effects of nano-and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 76:1356–1365
Xu P et al (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Yu JC, Tang HY, Yu JG (2002) Bacteriearal and photocatalytic activities of TiO2 thin films prepared by sol–gel and reverse micelle methods. J Photochem Photobiol a Chem 3:211–219
Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC (2007) Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 67:160–166
Zhao D, Wang J, Sun B, Sun B, Gao J, Xu R (2000) Development and application of TiO2 photocatalysis as antimicrobialagent. J Liaoning Univ (Nat Sci Ed) 2:173–174
Zhao J, Wang Z, Liu X, Xie X, Zhang K, Xing B (2011) Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. J Hazard Mater 197:304–310
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291:78–81
Zhu X, Chang Y, Chen Y (2010a) Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78:209–215
Zhu X, Wang J, Zhang X, Chang Y, Chen Y (2010b) Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79:928–933
Zhu X, Zhou J, Cai Z (2011) The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Poll Bull 63:334–338
Zhu X, Tian S, Cai Z (2012) Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One 7:e46286
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Khosravi-Katuli, K., Prato, E., Lofrano, G. et al. Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res 24, 17326–17346 (2017). https://doi.org/10.1007/s11356-017-9360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9360-3