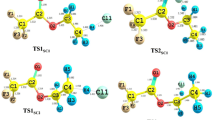
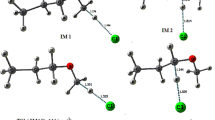
Abstract
The temperature-dependent rate coefficients were calculated for the reactions of Cl atoms with propene (R1), 1-butene (R2), 1-pentene (R3), and 1-hexene (R4) over the temperature range of 200–400 K. Canonical variational transition state theory (CVT) with small curvature tunneling (SCT) and conventional transition state theory (CTST) in combination with MP2/6-31G(d,p), MP2/6-31G+(d,p), and MP2/6–311 + G(d,p) level of theories were used to calculate the kinetic parameters. The obtained rate coefficients at 298 K for the reactions of Cl atoms with propene, 1-butene, 1-pentene, and 1-hexene are 1.36 × 10−10 cm3 molecule−1 s−1, 1.53 × 10−10 cm3 molecule−1 s−1, 4.61 × 10−10 cm3 molecule−1 s−1, and 4.76 × 10−10 cm3 molecule−1 s−1, respectively. In all these reactions, strong negative temperature dependence was observed over the studied temperature range. Cl atom addition across the double bond is the most dominant pathway. The contribution of abstraction channels towards their global rate coefficients was observed to be increasing from propene to 1-hexane. Atmospheric implications such as effective lifetimes and thermodynamic parameters of the test molecules were investigated in the present study.












Similar content being viewed by others
References
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103:4605–4638
Atkinson R (2000) Atmospheric chemistry of VOCs and NOx. Atmos Environ 34:2063–2101
Atkinson R (1997) Gas phase tropospheric chemistry of volatile organic compounds: 1. Alkanes and alkenes. J Phys Chem Ref Data 26:215–290
Atkinson R (1986) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 86:69–20
Bufalini JJ, Walter TA, Bufalini MM (1976) Ozone formation potential of organic compounds. Environ Sci Technol 10:908–912
Contos DA, Holdren MW, Smith DL, Brooke RC, Rhodes VL, Rainey ML (1995) Sampling and analysis of volatile organic compounds evolved during thermal processing of acrylonitrile butadiene styrene composite resins. J Air Waste Manage Assoc 45:686–694
Coquet S, Ariya PA (2000) Kinetics of the gas-phase reactions of cl atom with selected C2-C5 unsaturated hydrocarbons at 283 < T < 323 K. In J Chem Kinet 32:478–484
Curtiss LA, Redfern PC, Raghavachari K, Vitaly R, Pople JA (1999) Gaussian-3 theory using reduced Moller-Plesset order. J Chem Phys 110:4703–4709
Dash MR, Rajakumar B (2014) Reaction kinetics of cl atoms with limonene: an experimental and theoretical study. Atmos Environ 99:183–195
Espinosa-Garcia J (2003) Ab initio and variational transition state theory study of the CF3CF2OCH3 + OH reaction using integrated methods: mechanism and kinetics. J Phys Chem A 107:1618–1626
Ezell MJ, Wang W, Ezell AA, Soskin G, Finlayson-Pitts BJ (2002) Kinetics of reactions of chlorine atoms with a series of alkenes at 1 atm and 298 K: structure and reactivity. J Phys Chem Chem Phys 4:5813–5820
Finlayson-Pitts BJ, Pitts JN Jr (1986) Atmospheric chemistry: fundamentals and experimental techniques. Wiley-Interscience, New York
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2010) Gaussian 09, revision B.01. Gaussian, Inc., Wallingford
Galán E, González I, Fabbri B (2002) Estimation of fluorine and chlorine emissions from Spanish structural ceramic industries the case study of the Baillen area, southern Spain. Atmos Environ 36:5289–5298
Gonzalez-Lafont A, Truong TN, Truhlar DG (1991) Interpolated variational transition state theory: practical methods for estimating variational transition state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J Chem Phys 95:8875–8894
Graedel TE (1978) Chemical compounds in the atmosphere. Academic Press, New York
Kaiser EW, Wallington TJ (1996) Pressure dependence of the reaction cl+ C3H6. J Phys Chem 100:9788–9793
Keene WC. (1995) In Naturally produced organohalogens (eds A. Grimvall, E.W. B. de Leer) inorganic Cl cycling in the marine boundary layer: A Review. Academic Publishers, 363–373
Kent JA (2007) Kent and Riegel’s hand book of Industrial Chemistry and Biotechnology (11th edn, Vol. 1)
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Garrett BC, Steckler R, Isaacson AD, Rai SN (1992) G.C. POLYRATE 4. Comput Phys Commun 71:235–262
Lynch BJ, Truhlar DG (2001) How well can hybrid density functional methods predict transition state geometries and barrier heights? J Phys Chem A 105:2936–2941
McGillen MR, Percival CJ, Shalleross DE, Harvey JN (2007) Is hydrogen abstraction an important pathway in the reaction of alkenes with the OH radical? Phys Chem Chem Phys 9:4349–4356
Molina MJ, Molina LT, Kolb CE (1996) Gas phase and heterogeneous chemical kinetics of the troposphere and stratosphere. Annu Rev Phys Chem 47:327–367
Moller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Morris ED, Niki H (1971) Reactivity of hydroxyl radicals with olefins. J Phys Chem 75:3640–3641
Ohta T (1984) Rate constants for the reactions of OH radicals with alkyl substituted olefins. In J Chem Kinet 16:879–886
Orlando JJ, Tyndall GS, Apel EC, Riemer DD, Paulson SE (2003) Mechanisms of the reaction of cl atoms with a series of unsaturated hydrocarbons under atmospheric conditions. In J Chem Kinet 35:334–353
Pedro AB, Sordo JA (2003) Theoretical approach to the mechanism of reactions between halogen atoms and unsaturated hydrocarbons: the cl +propene reaction. J Comput Chem 24:2044–2062
Platt U, Allan W, Lowe D (2004) Hemispheric average cl atom concentration from 13C/12C ratios in atmospheric methane. Atmos Chem Phys 4:2393–2399
Rowland FS (1991) Stratospheric ozone depletion. Annu Rev Phys Chem 42:731–768
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of hartree-fock, møller-plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100:16502–16513
Singh HB, Thakur AN, Chen YE, Kanakidou M (1996) Tetrachloroethylene as an indicator of low cl atom in the troposphere. Geophys Res Lett 23:1529–1532
Singleton DL, Cvetonovoic RJ (1976) Temperature dependence of the reaction of oxygen atoms with olefins. J Am Chem Soc 98:6812–6819
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Unexpectedly high concentrations of molecular chlorine in coastal air. Nature 394:353
Thornton JA, Kercher JP, Riedel TP, Wagner NL, Cozic J, Holloway JS, Dubé WP, Wolfe GM, Quinn PK, Middlebrook AM, Alexander B, Brown SS (2010) A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature 464:271–274
Vijayakumar S, Rajakumar B (2016) Kinetic investigation of chlorine atom initiated photo oxidation reactions of 2,3-dimethyl-1,3-butadiene in the gas phase: an experimental and theoretical study. RSC Adv 6:67739–67750
Walavalkar MP, Sharma A, Alwe HD, Pushpa KK, Dhanya S, Naik PD, Bajaj PN (2013) Cl atom initiated oxidation of 1-alkenes under atmospheric conditions. Atmos Environ 67:93–100
Walavalkar MP, Vijayakumar S, Sharma A, Rajakumar B, Dhanya S (2016) Is H atom abstraction important in the reaction of cl with 1-alkenes? J Phys Chem A 120:4096–4107
Wert BP, Trainer M, Fried A, Ryerson TB, Henry B, Potter W, Angevine WM, Atlas E, Donnelly SG, Fehsenfeld FC et al (2003) Signatures of terminal alkene oxidation in airborne formaldehyde measurements during TexAQS 2000. J Geophys Res Atmos 108(D3):4104–4118
Yoon JW, Jhung SH, Lee JS, Kim TJ, Lee HD, Chang JS (2008) Effect of butadiene in catalytic trimerization of isobutene using commercial C4 feeds. Bull Kor Chem Soc 29:57–60
Zheng J, Zhang S, Corchado JC, Chuang YY, Coitiño EL, Ellingson BA, Truhlar DG (2010) GAUSSRATE, version 2009-A. University of Minnesota, Minneapolis
Zheng J, Zhang S, Lynch BJ, Corchado JC, Chuang YY, Fast PL, Hu WP, Liu YP, Lynch GC, Nguyen KA et al (2009) POLYRATE, version, vol 2008. University of Minnesota, Minneapolis
Acknowledgements
We gratefully acknowledge the Department of Science and Technology (DST), Government of India for their financial support and also we thank Mr.V. Ravichandran, High Performance Computing Environment (HPCE), IIT Madras for computer resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Electronic supplementary material
ESM 1
(DOCX 353 kb)
Rights and permissions
About this article
Cite this article
Vijayakumar, S., Rajakumar, B. Theoretical investigations on the kinetics of Cl atom initiated reactions of series of 1-alkenes. Environ Sci Pollut Res 25, 4387–4405 (2018). https://doi.org/10.1007/s11356-017-0638-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0638-2