Abstract
The control of filariasis vectors has been enhanced in several areas, but there are main challenges, including increasing resistance to insecticides and lack of cheap and eco-friendly products. The toxicity of iron (Fe0) and iron oxide (Fe2O3) nanoparticles has been scarcely investigated yet. We studied the larvicidal and pupicidal activity of Fe0 and Fe2O3 nanoparticles against Culex quinquefasciatus. Fe0 and Fe2O3 nanoparticles produced by green (using a Ficus natalensis aqueous extract) and chemical nanosynthesis, respectively, were analyzed by UV–Vis spectrophotometry, FT-IR spectroscopy, XRD analysis, SEM, and EDX assays. In larvicidal and pupicidal experiments on Cx. quinquefasciatus, LC50 of Fe0 nanoparticles ranged from 20.9 (I instar larvae) to 43.7 ppm (pupae) and from 4.5 (I) to 22.1 ppm (pupae) for Fe2O3 nanoparticles synthesized chemically. Furthermore, the predation efficiency of the guppy fish, Poecilia reticulata, after a single treatment with sub-lethal doses of Fe0 and Fe2O3 nanoparticles was magnified. Overall, this work provides new insights about the toxicity of Fe0 and Fe2O3 nanoparticles against mosquito vectors; we suggested that green and chemical fabricated nano-iron may be considered to develop novel and effective pesticides.
Similar content being viewed by others
Introduction
Mosquitoes constitute a major public health problem acting as vectors of serious diseases like malaria, filariasis, Japanese encephalitis, dengue fever, chikungunya, yellow fever, Zika virus, and others (Benelli and Mehlhorn 2016; Ashokan et al. 2017; Benelli and Romano 2017). Culex quinquefasciatus Say is a major vector of lymphatic filariasis, which affects 120 million people worldwide. Furthermore, about 400 million people are at risk of contracting filariasis, resulting in an annual economic loss of 1.5 billion dollars (WHO 2002, 2014; Vadivalagan et al. 2017). Cx. quinquefasciatus is also a potential vector of West Nile virus and Rift Valley fever virus (Farajollahi et al. 2011; Linthicum et al. 2016). Additionally, Cx. quinquefasciatus can transmit Japanese encephalitis virus, St. Louis encephalitis virus, reticuloendotheliosis virus Murray Valley encephalitis, and reovirus type 3 (Sakthivadivel et al. 2016).
The control of arthropods, with special reference to filarasis vectors, such as Cx. quinquefasciatus, as well as other mosquito species, has been enhanced in several areas, but there are main challenges, including increasing resistance to pesticides (Naqqash et al. 2016; Benelli and Beier 2017), massive non-target effects (Anyaele and Amusan 2003), and lack of cost-effective and eco-friendly products (Amer and Mehlhorn 2006a, b; Nathan et al. 2006; Rahuman et al. 2009; Benelli 2015a, b; Pavela and Benelli 2016; Banumathi et al. 2017).
Bionanoscience is a fast-growing research field nowadays (Kumar et al. 2010; Benelli and Lukehart 2017). Several approaches are available for the synthesis of nanoparticles. They include thermal decomposition (Navaladian et al. 2007), chemical reduction (Krishna and Dan 2009), photochemical reactions in reverse micelles (Taleb et al. 1997), electrochemical routes (Starowicz et al. 2006), microwave-assisted processes (Sreeram et al. 2008), and even green chemistry reduction routes (Begum et al. 2009; Ahmad et al. 2010; Raut et al. 2010; Iravani 2011). The latter can be done relying to the use of fungal and bacteria filtrates, as well as various plant extracts (Holmes et al. 1995; He et al. 2007; Nair and Pradeep 2002; Mukherjee et al. 2001; Saha et al. 2010; Shankar et al. 2004a, b; Saxena et al. 2010; Benelli 2016a, b). Green approaches provide noticeable advantages over chemical and physical methods, as they are cost-effective, do not need the use of highly toxic chemicals and can be easily scaled up for large-scale preparations. However, while a wide number of green fabrication routes have been carried out to produce Ag, ZnO, and Au nanoparticles with relevant toxicity against mosquitoes (Rajakumar and Rahuman 2011; Roni et al. 2015; Benelli et al. 2017a, b), the toxicity of iron and iron oxide nanoparticles against these important vectors has been scarcely investigated (Murugan et al. 2017).
Iron nanoparticles have attracted considerable interest due to their super paramagnetic properties and their potential biomedical applications arising from their biocompatibility and non-toxicity (Pankhurst et al. 2003). Therefore, iron nanoparticles have been used in magnetic resonance imaging, drug delivery, tissue repair, immunoassays, detoxification of biological fluids, hyperthermia and in cell separation (Vicky et al. 2010). The green biosynthesis of iron and iron oxide nanoparticles showing different sizes and shapes has been carried out using bacteria (Yeary et al. 2005), fungi (Roh and Moon 2006) as well as plant extracts (Senthil and Ramesh 2012). To the best of our knowledge, no studies focused on the toxicity of iron (Fe0) and iron oxide (Fe2O3) nanoparticles against mosquitoes. Therefore, here we investigated the synthesis of Fe0 and Fe2O3 nanoparticles through green and chemical reduction methods, respectively.
In several parts of the world, herbal preparations containing plant parts from Ficus species are used in traditional medicine, including for the treatment of malaria (Iwu 1993; Burkill 1997; Mandal et al. 2000; Titanji et al. 2008; Kuete et al. 2008, 2009; Chinsembu and Hedimbi 2010; Jansen et al. 2010). For the green synthesis of Fe0 nanoparticles, we selected a plant of phytochemical interest, the “mutuba tree” Ficus natalensis Hochst., an evergreen tree of 6–21 m high, with a rounded spread and dense crown (Rodriguez and Wrangham 1993; Woodland 1997; Adriens 2005). Despite the numerous traditional uses of this species, with special reference to the African region (Veale et al. 1992; Rabe and Van Staden 1997; Tabuti 2007), the potential of this plant as a green insecticide and reducing agent for nanosynthesis has not been tested.
Therefore, in this work, Fe0 and Fe2O3 nanoparticles produced by chemical and F. natalensis-mediated green nanosynthesis were analyzed by UV–Vis spectrophotometry, FT-IR spectroscopy, XRD analysis, SEM, and EDX assays. Both Fe0 and Fe2O3 nanoparticles were tested on larvae (instars from I to IV) and pupae of Cx. quinquefasciatus. Furthermore, we monitored the predation efficiency of a mosquito natural enemy, i.e., the guppy fish Poecilia reticulata Peters, after a single treatment with sub-lethal doses of Fe0 and Fe2O3 nanoparticles.
Materials and methods
Green synthesis of Fe0 nanoparticles
F. natalensis was collected from Coimbatore, Tamil Nadu, Southern India; the F. natalensis leaves were washed with tap water and shade-dried at 25 °C for 10 days. The F. natalensis extract was prepared as described by Dinesh et al. (2015), it was then filtered using Whatman No. 1, and stored at − 4 °C till nanosynthesis. In the green nanosynthesis process, the F. natalensis filtrate was mixed with an aqueous 1 mM Fe3O4 solution in an Erlenmeyer flask and incubated at 25 °C. A darker solution indicated the formation of iron nanoparticles, since aqueous Fe3+ was reduced to Fe0. Fe3O4 was purchased from Precision Scientific Co. (Coimbatore, India).
Chemical synthesis of Fe2O3 nanoparticles
One millimole of Fe(NO3)3.9 H2O was dissolved in 10 mL of double-distilled water, and 2 mmol of citric acid monohydrate was dissolved in 10 mL of double-distilled water. After 1 h, the acid solution was mixed into ferric solution and gently vortexed for 1 h. The homogeneous colloidal solution was poured in to a 40-mL Teflon flask hydrothermal autoclave and kept it in hot air oven at 180 °C for 12 h. The precipitated Fe2O3 nanoparticles were collected and washed with double-distilled water up to neutral pH. Then, the Fe2O3 nanoparticles were dried at 110 °C for 1 h and calcined for 2 h at 700 °C.
Characterization of Fe0 and Fe2O3 nanoparticles
The Fe0 and Fe2O3 nanoparticles were analyzed by UV–Vis spectrophotometry, FT-IR spectroscopy, XRD analysis, SEM, and EDX assays, following the methods described by Sujitha et al. (2015) and Qu et al. (2014), respectively. UV–Vis spectrophotometry was done at the wavelength of 200–800 nm in a UV-3600 Shimadzu spectrophotometer (1 nm resolution). SEM was carried out using a FEI Quanta 200 SEM. FTIR spectroscopy (Stuart 2002) was done with a Perkin-Elmer Spectrum 2000 FTIR spectrophotometer (Sujitha et al. 2015).
Toxicity against Cx. quinquefasciatus
A pathogen- and parasite-free strain of Cx. quinquefasciatus was originally established as described by Murugan et al. (2015d) in laboratory conditions [27 ± 2 °C; 7–85% R.H.; 14:10 (L:D)]. Eggs were kindly provided by the National Centre for Disease Control (NCDC) field station of Mettupalayam (Tamil Nadu). In the toxicity assays, 25 Cx. quinquefasciatus larvae (first, second, third, or fourth instar) or pupae were placed for 24 h in a glass beaker filled with 250 mL of dechlorinated water plus the desired concentration of the F. natalensis leaf extract, Fe0 or Fe2O3 nanoparticles, following the method by Mahesh Kumar et al. (2016), each concentration was replicated five times against all instars. Control mosquitoes were exposed for 24 h to clean water without Fe0 and Fe2O3 nanoparticles or the plant extract (Govindarajan et al. 2016a).
Impact of Fe0 and Fe2O3 nanoparticles on guppy predation
Guppies, P. reticulata, were collected from rural ponds in Coimbatore (India), identified by a taxonomist at the Department of Zoology of Bharathiar University (Coimbatore, India), and maintained in laboratory [27 ± 2 °C and 75–85% R.H.; 14:10 (L: D)] as reported by Murugan et al. (2015d). Guppies were studied for their predatory activity towards the II and III instar larvae of Cx. quinquefasciatus. For each tested instar, 400 mosquito larvae plus 1 adult P. reticulata were introduced in a plastic cup arena containing 5 L of dechlorinated water plus sub-lethal concentrations of Fe0 and Fe2O3 nanoparticles (tested concentration: 1/3 of the LC50 calculated on I instar Cx. quinquefasciatus larvae, see Murugan et al. 2015b). For predation in standard laboratory conditions, Fe0 and Fe2O3 nanoparticles were not added to the arenas containing guppy fishes and Cx. quinquefasciatus individuals. All cups were checked after 12 and 24 h (corresponding to night and day time) and the larvae consumed by guppies was recorded. Predated larvae were replaced with new ones from a same age cohort. To standardize the appetence of P. reticulata fishes, all tested fishes were food-deprived for 24 h before testing. Predatory efficiency of P. reticulata was calculated using the formula by Subramaniam et al. (2016).
Statistical analysis
Cx. quinquefasciatus larval and pupal mortality data were analyzed by probit analysis, calculating LC50 and LC90 values, 95% CI, regression equation, and chi squares following the method by Finney (1971). Chi squares were not significant (Benelli 2017). Guppy predation data were analyzed using a generalized linear model y = Xβ + ε described by Murugan et al. (2015b) with two fixed factors (i.e., treatment and targeted instar). P < 0.05 was used to assess the significance of differences among mean values.
Results and discussion
Characterization of iron nanoparticles
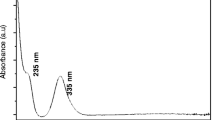
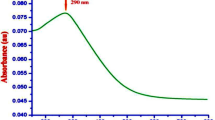
The UV–Vis spectrum of the Fe0 nanoparticles is provided in Fig. 1a, showing an absorption maxima at 255 nm in visible range between 200 to 800 nm wavelengths. The green synthesis of Fe nanoparticles was confirmed within 120 min after that the F. natalensis leaf extract was added to the Fe3O4 solution; the color changed from pale yellow to dark brown (Fig. 1b), and this probably arise from surface plasmon resonance of the nanostructures (Shankar et al., 2004a, b). The absorption spectrum of Fe2O3 nanoparticles showed a maximum absorption peak at 90 nm (Fig. 1c), at variance with the absorption maximum recently reported at 272 nm in the UV–Vis spectrum of Fe2O3 nanoparticles (Tharani and Nehru 2015). Earlier, it has been noted that the characteristic surface plasmon resonance band of Fe3O4 mostly occurred at wavelength within the range of 190–250 nm, as a function of different concentrations of metal ions with different volumes of plant extracts (Al-Kalifawi 2015). Also, Basavegowda et al. (2014a, b) reported comparable UV–Vis spectra for Fe3O4 nanoparticles synthesized using extracts of Artemisia annua and Perilla frutescens.
a UV–Vis spectrum of iron nanoparticles green synthesized using the Ficus natalensis leaf extract. b This panel shows the color changes before and after the process of reduction of Fe3+ to Fe0 nanoparticles. c UV–Vis absorption spectrum of the Fe2O3 nanoparticles chemically synthesized; both spectra were recorded after 120 min from the start of the reaction
The XRD pattern of Fe2O3 nanoparticles is showed in Fig. 2a, contains a number of peaks, which are clearly distinguishable (Fu et al. 2001). The obtained data matched with the Joint Committee on Powder Diffraction Standards (JCPDS) File No. (87-1166). The fact indicates that the nanoparticles Fe2O3 crystals may develop preferentially rather than randomly (Joya et al. 2013). Figure 2b showed the phase purity and crystallinity of Fe0 nanoparticles. XRD pattern showed intense peaks corresponding to the (111), (200), (311), (222), and (220) sets of lattice planes. The sharp Bragg peaks reported above might also result from capping agents stabilizing the iron nanoparticles (see Chen et al. 2009; Farrukh et al. 2013).
SEM was used to confirm the morphology of the Fe2O3 nanoparticles (Fig. 3a), which were spherical in shape, while SEM observations on green synthesized Fe0 nanoparticles showed different shapes, including rod-like structures, with size ranging from 35 to 40 nm (Fig. 3b). Concerning other syntheses of iron nanoparticles, it has been elucidated that Fe3O4 synthesized by co-precipitation with different reagents (FeCl3.6H2O, FeCl2.4H2O, propylene glycol, and ammonium hydroxide) had mean size of 8 nm (Shen et al. 2009). Mono-dispersity control is very important since the properties of nanocrystals strongly depend to the dimension of nanoparticles (Sophie et al. 2008).
Figure 4a–b shows EDX strong peaks for Fe and O confirming their presence in the formation of iron and Fe2O3 nanoparticles. In agreement with our work, Hariani et al. (2013) reported strong peaks of Fe and O (see also Noruzi et al. 2012). The composition of Fe2O3 nanoparticles formed by co-precipitation synthesis was Fe 73.36% and O 21.02%. The EDX spectrum recorded from iron nanoparticles showed a distinct signal and high atomic percent values for iron (Fig. 4b).
Figure 5a showed the FT-IR spectrum of Fe2O3 nanoparticles with peaks at 3138, 1645, 1401, 894, and 790 cm−1, denoting stretching in O–H, C=O, and C–O bonds of carboxylic acids and 850-out-of-plane C–H vibration, respectively (Al-Bawabe et al. 1998; Srinivas et al. 2013). Green fabricated iron nanoparticles showed a main peak at 3450.80 (Fig. 5b) as well as some peaks decreasing in intensity at 2313.71, 530.45, and 450.45 cm−1, the presence of active functional groups in this extract can play a role for the swift reduction of iron ions to iron nanoparticles (Yew et al. 2016). Similarly, Yuvakkumar and Hong (2014) reported the presence of phenolic compounds and proteins responsible for the formation and stabilization of synthesized iron oxide nanoparticles.
Toxicity against Cx. quinquefasciatus
The F. natalensis leaf extract showed limited larvicidal and pupicidal properties on Cx. quinquefasciatus; LC50 values ranged from 234.6 ppm (I instar larvae), 259.0 ppm (II instar), 317.7 ppm (III instar), 400.1 ppm (IV instar), and 504.1 (pupae) ppm, respectively (Table 1); a dose-dependent effect was found, as reported for a growing number of botanicals recently tested against mosquito vectors (Amer and Mehlhorn 2006b, Dinesh et al. 2015; Murugan et al. 2015a, b, c; Govindarajan and Benelli 2016). Concerning the genus Ficus, Chung et al. (2011) reported that the milky sap of F. carica has a toxic effect against early fourth stage larvae of Aedes aegypti with an LC50 value of 10.2 µg/mL and an LC90 value of 42.3 µg/mL (Chung et al. 2011).
Furthermore, in larvicidal and pupicidal experiments conducted testing Fe0 and Fe2O3 nanoparticles on Cx. quinquefasciatus, LC50 values of green fabricated iron nanoparticles ranged from 20.9 (I instar larvae) to 43.7 ppm (pupae) (Table 2), while they were from 4.5 (I) to 22.1 ppm (pupae) for Fe2O3 nanoparticles synthesized chemically (Table 3). To the best of our knowledge, the toxic potential of Fe0 and Fe2O3 nanoparticles against mosquito vectors has not been investigated yet, with the only exception of a very recent study by Murugan et al. (2017), where magnetic nanoparticles from the magnetosomes of Magnetospirillum gryphiswaldense were tested against A. aegypti mosquitoes and as growth inhibitor of dengue virus serotype DEN-2.
On the other hand, a wide number of studies have investigated the potential of silver, gold, and ZnO nanoparticles against various mosquito species as well as microbial pathogens (Arokiyaraj et al. 2013; Pavela and Benelli 2016; Benelli et al. 2017b). Ag nanoparticles produced using a green method based on the employ of the aqueous bark extract of Ficus racemosa also showed high larvicidal activity against mosquitoes (Velayutham et al. 2013). Besides, it should be noted that the toxicity of the iron and iron oxide nanoparticles tested in the present research is lower if compared to some selected plant essential oils, as pointed out by Pavela (2015a), who highlighted the toxicity of seven essential oils with larvicidal LC50 lower than 10 ppm. Later, Govindarajan and Benelli (2016), Alshebly et al. (2017), Govindarajan et al. (2017), and Benelli et al. (2017c) isolated several molecules (e.g., ar-curcumene, (4E,6Z)-allo-ocimene and carvotanacetone) from medicinal plant essential oils showing larvicidal LC50 values lower than 8 ppm, while Pavela (2015b) and Benelli et al. (2017d) shed light on synergistic effects of selected plant essential oils formulated in highly effective larvicidal blends against Cx. quinquefasciatus.
The mechanism of action of iron and iron oxide nanoparticles against mosquitoes, as well as on other arthropod vectors, still needs to be clarified, as recently stressed also for silver, gold, titania, and zinc oxide nanoparticles (Benelli et al. 2017e), while it has been observed that the toxicity exerted by magnetic nanoparticles against DEN-2 virus is partially due to inhibiting the expression of the envelope (E) protein (Murugan et al. 2017).
Impact of Fe0 and Fe2O3 nanoparticles on guppy predation
In standard laboratory assays, after 24 h, P. reticulata predation rates towards II and III instar larvae of Cx. quinquefasciatus were 62.7 (II) and 45.5% (III) (Table 4). II instar larvae were probably preferred by P. reticulata because of their smaller size thus reduced mobility. Post-treatment with Fe0 nanoparticles, P. reticulata predation was boosted to 75.4% on II instar larvae, and 59.0% on III instar larvae (Table 4), respectively. Post-treatment with Fe2O3 nanoparticles, predation was boosted to 79.2 and 61.0%, respectively (Table 4). The enhanced predation rates of guppies on Cx. quinquefasciatus’ younger larvae may be due to a higher impact of nanoparticles treatment on mosquito physiological and metabolic activities, leading to higher motility reduction in II instar larvae over to III instar ones, as previously discussed by Murugan et al. (2015b, e). Besides, no detectable toxicity was observed on P. reticulata individuals exposed to Fe0 and Fe2O3 nanoparticles over a week (data not shown).
A number of recent studies supported the eco-friendly nature of green-synthesized nanoparticles used for mosquito control, since—at the doses used to fight mosquito young instars—they showed no or limited toxicity against non-target species, including mosquito natural enemies (Patil et al. 2012a, b; Haldar et al. 2013; Rawani et al. 2013b; Murugan et al. 2015c; Ramanibai and Velayutham 2015; Mahesh Kumar et al. 2016). However, the employ of nanoformulated metals and metal oxides in the aquatic environment is still debated and more knowledge in needed about potential long-term effects due to the exposure to nanoparticles, including risks of genotoxicity (Benelli et al. 2017b).
Conclusions
Overall, even if the control of filariasis mosquito vectors has been ameliorated in several areas, there are main challenges, including increasing resistance to insecticides and lack of cost-effective and eco-friendly products. In the present investigation, we discovered the high larvicidal and pupicidal activity of Fe0 and Fe2O3 nanoparticles testing them against Cx. quinquefasciatus. Notably, the predation efficiency of the guppy fish, P. reticulata, after a single treatment with sub-lethal doses of Fe0 and Fe2O3 nanoparticles, was boosted. In conclusion, this work provides new insights report about the toxicity of Fe0 and Fe2O3 nanoparticles against mosquito vectors. Therefore, we argued that green- and chemical-fabricated Fe0 and Fe2O3 nanoparticles may be considered to develop novel and effective pesticides.
References
Adriens M (2005) Family medicinal plant gardens in Rwenzori region, 1st edn. Marianum press Ltd., Kisubi
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces 81:81–86
Al-Bawabe A, Friberg SE, Sjoblom J, Farrington G (1998) Sol/Gel Glass with Ferric Nitrate Hydrate Temperature Dependence Transition II high concentration of iron in the glass. J Disp Sci Technol 19:613–636
Al-Kalifawi E (2015) Green synthesis Of Magnetite Iron Oxide Nanoparticles by Using Al-Abbas's (AS) Hund Fruit (Citrus medica) var Sarcodactylis Swingle Extract and Used in Al-'alqami River Water Treatment. J Nat Sci Res 5:20
AlShebly MM, AlQahtani FS, Govindarajan M, Gopinath K, Vijayan P, Benelli G (2017) Toxicity of ar-curcumene and epi-β-bisabolol from Hedychium larsenii (Zingiberaceae) essential oil on malaria, chikungunya and St. Louis encephalitis mosquito vectors. Ecotoxicol Environ Saf 137:149–157
Amer A, Mehlhorn H (2006a) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Amer A, Mehlhorn H (2006b) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Anyaele OO, Amusan AAS (2003) Toxicity of hexanoic extracts of Dennettia tripetala (G. Baxer) on larvae of Aedes aegypti (L). Afr J Biomed Res 6:49–53
Arokiyaraj S, Saravanan M, Udaya Prakash NK (2013) Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L leaf extract: an in vitro study. Mat Res Bull 9:3323–3327
Ashokan AP, Paulpandi M, Dinesh D, Murugan K, Vadivalagan C, Benelli G (2017) Toxicity on dengue mosquito vectors through Myristica fragrans-Synthesized zinc oxide nanorods, and their cytotoxic effects on liver cancer cells (HepG2). J Clust Sci 28:205–226
Banumathi B, Vaseeharan B, Periyannan R, Prabhu NM, Ramasamy P, Murugan K, Canale A, Benelli G (2017) Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick, Rhipicephalus (Boophilus) microplus – a review. Vet Parasitol. https://doi.org/10.1016/j.vetpar.2017.07.021
Basavegowda N, Magar KBS, Mishra K, Lee YR (2014a) Green fabrication of ferromagnetic Fe3O4 nanoparticles and their novel catalytic applications for the synthesis of biologically interesting benzoxazinone and benzthioxazinone derivatives. New J Chem 38(11):5415–5420
Basavegowda N, Mishra K, Lee YR (2014b) Sonochemically synthesized ferromagnetic Fe3O4 nanoparticles as a recyclable catalyst for the preparation of pyrrolo [3, 4-c] quinoline-1, 3-dione derivatives. RSC Adv 4(106):61660–61666
Begum NA, Mondal S, Basu S, Laskar RA, Mandal D (2009) Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf B: Biointerfaces 71(1):113–118
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114(9):3201–3212
Benelli G (2016a) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2016b) Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—a brief review. Enzym Microb Technol 95:58–68
Benelli G (2017) Commentary: data analysis in bionanoscience-issues to watch for. J Clust Sci 28:11-14
Benelli G, Beier J (2017) Current vector control challenges in the fight against malaria. Acta Trop 174:91–96
Benelli G, Lukehart CM (2017) Special issue: applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J Clust Sci 28:1–2
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Romano D (2017) Mosquito vectors of Zika virus. Entomol Gen. https://doi.org/10.1127/entomologia/2017/0496
Benelli G, Govindarajan M, Rajeswary M, Senthilmurugan S, Vijayan P, Alharbi NS, Kadaikunnan S, Khaled JM (2017a) Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: the promising potential of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitol Res 116:1175–1188
Benelli G, Maggi F, Pavela R, Murugan K, Govindarajan M, Vaseeharan B, Petrelli R, Cappellacci L, Kumar S, Hofer A, Youssefi MR, Alarfaj AA, Hwang JS, Higuchi A (2017b) Mosquito control with green nanopesticides: towards the One Health approach? A review of non-target effects. Environ Sci Poll Res. https://doi.org/10.1007/s11356-017-9752-4
Benelli G, Maggi F, Romano D, Stefanini C, Vaseeharan B, Kumar S, Higuchi A, Alarfaj AA, Mehlhorn H, Canale A (2017c) Nanoparticles as effective acaricides against ticks – a review. Ticks Tick-Borne Dis. https://doi.org/10.1016/j.ttbdis.2017.08.004
Benelli G, Pavela R, Canale A, Cianfaglione K, Ciaschetti G, Conti F, Nicoletti M, Senthil-Nathan S, Mehlhorn H, Maggi F (2017d) Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: synergistic and antagonistic effects. Parasitol Int 66:166–171
Benelli G, Pavela R, Maggi F, Petrelli R, Nicoletti M (2017e) Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J Clust Sci 28:3–10
Burkill HM (1997) The useful plants of west tropical Africa Royal Botanic Gardens. Kew 4(515):184–185
Chen CJ, Lai HY, Lin CC, Wang JS, Chiang RK (2009) Preparation of monodisperse iron oxide nanoparticles via the synthesis and decomposition of iron fatty acid complexes. Nanoscale Res Lett 4(11):1343–1350
Chinsembu KC, Hedimbi M (2010) An ethnobotanical survey of plants used to manage HIV/AIDS opportunistic infections in Katima, Caprivi region, Namibia. J Ethnobiol Ethnomed 6:25
Chung IM, Kim SJ, Yeo MA, Park SW, Moon HI (2011) Immunotoxicity activity of natural furocoumarins from milky sap of Ficus carica L against Aedes aegypti L. Immunopharmacol Immuno Toxicol 33(3):515–518
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1152
Farajollahi A, Fonseca DM, Kramer LD, Marm KA (2011) Bird biting mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infec Genet Evol 11:1577–1585
Farrukh MA, Ali S, Rahman MK (2013) Photodegradation of 2, 4, 6-trinitrophenol catalyzed by Zn/MgO nanoparticles prepared in aqueous-organic medium. Korean J Chem Eng 30:2100–2107
Finney DJ (1971) Probit analysis. Cambridge University Press, London
Fu Y, Chen J, Zhang H (2001) Chem Phys Lett 350:491
Govindarajan M, Benelli G (2016) Artemisia absinthium-borne compounds as novel larvicides: effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol Res 115:4649–4661
Govindarajan M, Khater HF, Panneerselvam C, Benelli G (2016a) One-pot fabrication of silver nanocrystals using Nicandra physalodes: a novel route for mosquito vector control with moderate toxicity on non-target water bugs. Res Vet Sci 107:95–101
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Khater HF, Benelli G (2016b) Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: a potent eco-friendly tool against malaria and arbovirus vectors. J Photochem Photobiol B Biol 161:482–489
Govindarajan M, Rajeswary M, Senthilmurugan S, Vijayan P, Alharbi NS, Kadaikunnan S, Khaled JM, Benelli G (2017) Curzerene, trans-β-elemenone and 훾-elemene as effective larvicides against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus: toxicity on non-target aquatic predators. Environ Sci Poll Res. https://doi.org/10.1007/s11356-017-8822-y
Haldar KM, Haldar B, Chandra G (2013) Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall). Parasitol Res 112:1451–1459
Hariani PL, Faizal R, Marsi D, Setiabudidaya M (2013) Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int J Environ Sci Dev 4:3
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61:3984–3987
Holmes JD, Smith PR, Evans-Gowing R, Richardson DJ, Russel DA, Sodeau JR (1995) Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol 163(2):143–147
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Iwu MM (1993) Handbook of African medicinal plants. CRC Press, LLC, London, pp 12–57
Jansen O, Angenot L, Tits M, Nicolas JP, De Mol P, Nikiema JB, Frederich M (2010) Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnophamacol 13:143–150
Joya MR, Baron-Jaimez J, Barba-Ortega J (2013) Preparation and characterization of F e2O3 nanoparticles. J Phys Conf Ser 466(2013):012004. https://doi.org/10.1088/1742-6596/466/1/012004
Krishna B, Dan VGJ (2009) Silver nanoparticles for printable electronics and biological applications. J Mater Res 24:2828–2836
Kuete V, Ngameni B, Fotso SCC, Kengap TR, Ngadjui BT, Meyer JJM, Lall N, Kuiate JR (2008) Antimicrobial activity of the crude extracts of and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae). J Ethnopharmacol 120:17–24
Kuete V, Nana F, Ngameni B, Mabveng TA, Keumedjio F, Ngadjui BT (2009) Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J Ethnopharmacol 124:556–561
Kumar R, Sharon M, Choudhary AK (2010) Nanotechnology in agricultural diseases and food safety. J Phytol 2:83–92
Linthicum KJ, Britch SC, Anyamba A (2016) Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol 61:395–415
Mahesh Kumar P, Murugan K, Madhiyazhagan P, Kovendan K, Amerasan D, Chandramohan B, Dinesh D, Suresh U, Nicoletti M, Saleh Alsalhi M, Devanesan S, Wei H, Kalimuthu K, Hwang JS, Lo Iacono A, Benelli G (2016) Biosynthesis, characterization and acute toxicity of Berberis tinctoria fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol Res 115:751–759
Mandal SC, Saha BP, Pa M (2000) Study on antimicrobial activity of Ficus racemosa Linn leaf extract. Phytother Res 14:278–280
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parischa R, Ajayakumar PV, Alam M, Kumar R, Sastry M (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 1:515–519
Murugan K, Benelli G, Ayyappan S, Dinesh D, Panneerselvam C, Nicoletti M, Hwang JS, Mahesh Kumar P, Subramaniam J, Suresh U (2015a) Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol Res 114:2243–2253
Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D, Nicoletti M, Hwang JS, Suresh U, Madhiyazhagan P (2015b) Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol 153:129–138
Murugan K, Eugine Venus JS, Panneerselvam C, Bedini S, Conti B, Nicoletti M, Kumar Sarkar S, Hwang JS, Subramaniam J, Madhiyazhagan P, Mahesh Kumar P, Dinesh D, Suresh U, Benelli G (2015c) Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environ Sci Pollut Res 21:17053–17064
Murugan K, Priyanka V, Dinesh D, Madhiyazhagan P, Panneerselvam C, Subramaniam J, Suresh U, Chandramohan B, Roni M, Nicoletti M, Alarfaj AA, Higuchi A, Munusamy MA, Khater HF, Messing RH, Benelli G (2015d) Predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector, Aedes aegypti, in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol Res 114:3601–3610
Murugan K, Sanoopa CP, Madhiyazhagan P, Dinesh D, Subramaniam J, Panneerselvam C, Roni M, Suresh U, Nicoletti M, Alarfaj AA, Munusamy MA, Higuchi A, Kumar S, Perumalsamy H, Ahn JY, Benelli G (2015e) Rapid biosynthesis of silver nanoparticles using Crotalaria verrucosa leaves against the dengue vector Aedes aegypti: what happens around? An analysis of dragonfly predatory behavior after exposure at ultra-low doses. Nat Prod Res 30:826–833
Murugan K, Wei J, Saleh Alsalhi M, Nicoletti M, Paulpandi M, Samidoss CM, Dinesh D, Chandramohan B, Paneerselvam C, Subramaniam J, Vadivalagan C, Wei H, Amuthavalli P, Jaganathan A, Devanesan S, Higuchi A, Kumar S, Aziz AT, Nataraj D, Vaseeharan B, Canale A, Benelli G (2017) Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol Res 116:495–502
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2:293–298
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Nathan SS, Chung PG, Murugan K (2006) Combined effect of biopesticides on the digestive enzymatic profiles of Cnaphalocrocis medinalis (Guenée) (the rice leaf folder) (Insecta: Lepidoptera: Pyralidae). Ecotoxicol Environ Saf 64:82–89
Navaladian S, Viswanathan B, Viswanath RP, Varadarajan TK (2007) Thermal decomposition as route for silver nanoparticles. Nanoscale Res Lett 2:44–48
Noruzi M, Zare D, Davoodi D (2012) A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 94:84–88
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36:167–181
Patil CD, Borase HP, Patil SV, Salunkhe RB, Salunke BK (2012a) Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol Res 111:555–562
Patil CD, Patil SV, Borase HP, Salunke BK, Salunkhe RB (2012b) Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol Res 110:1815–1822
Pavela R (2015a) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Pavela R (2015b) Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol Res 114:3835–3853
Pavela R, Benelli G (2016) Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci 21:1000–1007
Qu D, Zheng M, Zhang L, Zhao H, Xie Z, Jing X, Hadda RE, Fan H, Sun Z (2014) Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci Rep 4:5294
Rabe T, Van Staden J (1997) Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol 56:81–87
Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G (2009) Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 104:1365–1372
Rajakumar G, Rahuman AA (2011) Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vector. Acta Trop 118:196–203
Ramanibai R, Velayutham K (2015) Bioactive compound synthesis of Ag nanoparticles from leaves of Melia azedarach and its control for mosquito larvae. Res Vet Sci 98:82–88
Raut RW, Niranjan K, Kolekar N, Lakkakula J, Mendhulkar V, Kashid B (2010) Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L) Pierre. Nano Micro Lett 2:106–113
Rawani A, Ghosh A, Chandra G (2013) Mosquito larvicidal and anti-microbial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L (Solanaceae: Solanales). Acta Trop 128:613–622
Rodriguez E Wrangham R (1993) Zoopharmacognosy: the use of medicinal plants by animals. In: Phytochemical potential of tropical plants (pp. 89-105). Springer USA
Roh, Vali H, Phelps TJ, Moon JW (2006) Extracellular synthesis of magnetite and metal-substituted magnetite nanoparticles. J Nanosci Nanotechnol 6:3517–3520
Roni M, Murugan K, Panneerselvam C, Subramaniam J, Nicoletti M, Madhiyazhagan P, Dinesh D, Suresh U, Khater HF, Wei H, Canale A, Alarfaj AA, Munusamy MA, Higuchi A, Benelli G (2015) Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol Environ Saf 121:31–38
Saha S, Sarkar J, Chattopadhyay D, Patra S, Chakraborty A, Acharya K (2010) Production of silver nanoparticles by a phytopathogenic fungus Bipolaris nodulosa and its antimicrobial activity. Dig J Nanomater Biostruct 5(4):887–895
Sakthivadivel M, Saravanan T, Tenzin G, Jayakumar M, Raveen R (2016) Laboratory evaluation of two Meliaceae species as Larvicides against Culex quinquefasciatus Say (Diptera: Culicidae). Vector Biol J 1(2):2–10
Saxena A, Tripathi RM, Singh RP, Dig J (2010) Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Nanomat Bios 5(2):427–432
Senthil M, Ramesh C (2012) Biogenic synthesis of Fe3O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Digest J Nanomat Biostruct 7:1655–1660
Shankar SS, Rai A, Ahmad A, Sastry M (2004a) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275(2):496–502
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004b) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482–488
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo L (2009) Preparation and application of magnetic nanoparticles Fe3O4 for water purification. Sep Purif Technol 68:312–319
Sophie L, Delphine F, Marc P, Alain R, Caroline R, Luce VE, Robert NM (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterization, and biological applications. Chem Rev 108:2064–2110
Sreeram KJ, Nidin M, Nair BU (2008) Microwave assisted template synthesis of silver nanoparticles. Bull Mater Sci 31:937–942
Srinivas NL, Paul MK, Sree Vennela P, Venkata RD (2013) Green synthesis of silver nanoparticles using strawberry leaf extract (Arbutus unedo) and evaluation of its antimicrobial activity a novel study. Int J Nanomat Biostruct 3(3):47–50
Starowicz M, Stypuła B, Banaś J (2006) Electrochemical synthesis of silver nanoparticles. Electrochem Commun 8:227–230
Stuart BH (2002) Polymer analysis. John Wiley & Sons, London
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Dinesh D, Mahesh Kumar P, Chandramohan B, Suresh U, Rajaganesh R, Saleh AlSalhi M, Devanesan S, Nicoletti M, Canale A, Benelli G (2016) Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ Sci Poll Res 23:7543–7558
Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, Nicoletti M, Higuchi A, Madhiyazhagan P, Subramaniam J, Dinesh D, Vadivalagan C, Chandramohan B, Alarfaj AA, Munusamy MA, Barnard DR, Benelli G (2015) Green synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res 114:3315–3325
Tabuti JR (2007) The uses, local perceptions and ecological status of 16 woody species of Gadumire Sub-county, Uganda. Biodivers Conserv 16:1901–1915
Taleb A, Petit C, Pileni MP (1997) Synthesis of highly monodisperse silver nanoparticles from AOT reverse micelles: a way to 2D and 3D self-organization. Chem Mater 9:950–959
Tharani K, Nehru LC (2015) Synthesis and characterization of iron oxide nanoparticle by precipitation method. Int J Rec Adv Phys Sci 2:47–50
Titanji VPK, Zofou D, Ngemenya MN (2008) The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. AJTCAM 5:302–321
Vadivalagan C, Karthika P, Murugan K, Panneerselvam C, Del Serrone P, Benelli G (2017) Exploring genetic variation in haplotypes of the filariasis vector Culex quinquefasciatus (Diptera: Culicidae) through DNA barcoding. Acta Trop 169:43–50
Veale DJH, Furman KI, Oliver DW (1992) South African traditional herbal medicines used during pregnancy and childbirth. J Ethnopharmacol 36:185–191
Velayutham K, Rahuman AA, Rajakumar G, Mohan Roopan S, Elango G, Kamaraj C, Marimuthu S, Santhoshkumar T, Iyappan M, Siva C (2013) Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac J Trop Med 6:95–101
Vicky M, Rodney S, Ajay S, Hardik M (2010) Introduction to metallic nanoparticles. J Pharm Bioallied Sci 2:282–289
WHO (2002) Lymphatic filariasis, the disease and its control. Technical Report 71 WHO, Geneva
WHO (2014) Lymphatic filariasis. Fact sheet no 102
Woodland DW (1997) Contemporary plant systematics, 2nd edn. Press Berien Springs MI, Andrews University
Yeary LW, Ji WM, Love LJ, Thompson JR, Rawn CJ, Phelps TJ (2005) Magnetic properties of biosynthesized magnetite nanoparticles. Magn IEEE Trans 41:4384–4389
Yew YP, Shameli K, Miyake M, Kuwano N, Khairudin ANB, Mohamad SE, Lee KX (2016) Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res Lett 11:276
Yuvakkumar R, Hong SI (2014) Green synthesis of spinel magnetite iron oxide nanoparticles. Adv Mater Res 1051:39–42
Acknowledgements
Four anonymous reviewers improved an earlier version of our work. The authors are grateful to the Professor and Head, Department of Zoology, Bharathiar University for the laboratory facilities providing for this experiment. D. Dinesh is grateful to the Rajiv Gandhi National Fellowship, University Grant Commissions, New Delhi, India for the financial support. Project File No. F117.1/201617/RGNF201517SCTAM27906/ (SAIII/Website).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Murugan, K., Dinesh, D., Nataraj, D. et al. Iron and iron oxide nanoparticles are highly toxic to Culex quinquefasciatus with little non-target effects on larvivorous fishes. Environ Sci Pollut Res 25, 10504–10514 (2018). https://doi.org/10.1007/s11356-017-0313-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0313-7