Abstract
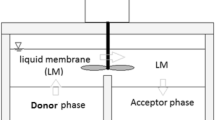
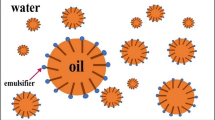
The removal of thallium ions in flue gas desulfurization wastewater from ferrous metallurgic industry was studied by emulsion liquid membrane (ELM) method using 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (P507) as carrier, aviation kerosene (AK) as organic solvent, polyisobutylene succinimide (T154) as surfactant, polyisobutylene (PIB) as additive, and sulfuric acid as internal reagent. Some important influence parameters such as concentrations of carrier, surfactant and stripping agent, agitation speed, extraction time, volume ratios of feed solution to emulsion phase and internal phase to membrane phase, and their effects on the removal efficiency of Tl in the ELM process were investigated and optimized. Under the optimum operating conditions of 2% of carrier, 5% of surfactant, 0.5 M of stripping agent, 350 rpm of agitation speed, 12.5:1 of volume ratio of feed solution to emulsion phase, and 3:1 volume ratio of membrane to internal phase, the maximum extraction efficiency of thallium reached 99.76% within 15-min reaction time. The ICP-MS analysis indicated that the thallium concentration in treated wastewater was below 5 μg/L and could meet the emission standard demand for industrial wastewater enacted by the local government of Hunan province of China. Meanwhile, the extraction of impurity ions calcium and magnesium in the ELM system was investigated. The result showed that an acidic environment would be in favor of the removal of Tl from calcium and magnesium contained in wastewater.

ᅟ








Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Albert L., Masson H.,(1992). Thallium extraction process. U.S. Patent 5296204
Anitha M, Ambare DN, Singh DK, Singh H, Mohapatra PK (2015) Extraction of neodymium from nitric acid feed solutions using an emulsion liquid membrane containing TOPO and DNPPA as the carrier extractants. Chem Eng Res Des 98:89–95
Cvjetko P, Cvjetko I, Pavlica M (2010) Thallium toxicity in humans. Arh Hig Rada Toxicol 61:111–119
Dâas A, Hamdaoui O (2014) Removal of non-steroidal anti-inflammatory drugs ibuprofen and ketoprofen from water by emulsion liquid membrane. Environ Sci Pollut Res 21:2154–2164
Das AK, Chakraborty R, Cervera ML, Guardia MDL (2006) Determination of thallium in biological samples. Anal Bioanal Chem 385:665–670
Das C, Rungta M, Arya G, DasGupta S, De S (2008) Removal of dyes and their mixture from aqueous solution using liquid emulsion membrane. J Hazard Mater 159:365–371
Du YC, Fan HG, Wang LP, Liu MH, Yang P (2012) Treatment on containing thallium wastewater by ECS activation and adsorption purification of microporous materials. Non-Metallic Mines 35:62–65 (in Chinese)
Dutrizac JE (1997) The behavior of thallium during jarosite precipitation. Metall Mater Trans B Process Metall Mater Process Sci 28:765–776
Dutrizac JE, Chen TT, Beauchemin S (2005) The behaviour of thallium (III) during jarosite precipitation. Hydrometall 79:138–153
Gallego-Lizon T, Pérez de Ortiz ES (2000) Drop sizes in liquid membrane dispersions. Ind Eng Chem Res 39:5020–5028
Galván-Arzate S, Santamaria A (1998) Thallium toxicity. Toxicol Lett 99:1–13
García MG, Acosta AO, Marchese J (2013) Emulsion liquid membrane pertraction of Cr (III) from aqueous solutions using PC-88A as carrier. Desalination 318:88–96
Herrero F, Fernandez E, Gomez J, Pretel L, Canizares F, Frias J, Escribano JB (1995) Thallium poisoning presenting with abdominal colic, paresthesia, and irritability. Clin Toxicol 33:261–264
Jia YL, Xiao TF, Zhou GZ, Ning ZP (2013) Advance on the chemical speciation of thallium in water, soil and sediment. Environ Chem 32:917–925 (in Chinese)
Kaghazchi T, Kargari A, Yegani R, Zare A (2006) Emulsion liquid membrane pertraction of l-lysine from dilute aqueous solutions by D2EHPA mobile carrier. Desalination 190:161–171
Krakowiak KE, Tarbet BJ, An H, Johnson DF, Bruening RL, (1995) Processes for removing, separating, and concentrating lead, thallium, alkali metals, alkaline earth metals from concentrated matrices using macrocyclic polyether cryptand ligands bonded to inorganic supports. U.S. Patent 5393892
Kulkarni PS, Mahajani VV (2002) Application of liquid emulsion membrane (LEM) process for enrichment of molybdenum from aqueous solutions. J Membr Sci 201:123–135
Kumbasar RA (2010a) Selective extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using tributhylphosphate as carrier. J Hazard Mater 178:875–882
Kumbasar RA (2010b) Selective extraction and concentration of chromium (VI) from acidic solutions containing various metal ions through emulsion liquid membranes using Amberlite LA-2. J Ind Eng Chem 16:829–836
Lee SC (2011) Extraction of succinic acid from simulated media by emulsion liquid membranes. J Membr Sci 381:237–243
Ma RJ (2009). Extraction metallurgy, In: SL Cao (Ed.) Metallurgical Industry Press, Beijing
Meggs WJ, Hoffman RS, Shih RD, Weisman RS, Goldfrank LR (1994) Thallium poisoning from maliciously contaminated food. Clin Toxicol 32:723–730
Memon SQ, Memon N, Solangi AR, Memon JR (2008) Sawdust: a green and economical sorbent for thallium removal. Chem Eng J 140:235–240
Moeschlin S., (1980). Thallium poisoning. Clin Toxicol 17, 133–146
Mortaheba HR, Kosugeb H, Mokhtarania B, Aminia MH, Banihashemia HR (2009) Study on removal of cadmium from wastewater by emulsion liquid membrane. J Hazard Mater 165:630–636
Mulkey JP, Oehme FW (1993) A review of thallium toxicity. Vet Hum Toxicol 35:445–453
Pan BC, Wan SL, Zhang SJ, Guo QW, Xu ZC, Lu L, Zhang WM (2014) Recyclable polymer-based nano-hydrous manganese dioxide for highly efficient Tl(I) removal from water. Sci Chin Chem 57:763–771
Paulo FMMC, Carvalho JMRD (2000) Recovery of 2-chlorophenol form aqueous solutions by emulsion liquid membranes batch experimental studies and modelling. J Membr Sci 179:175–183
Peter AL, Viraraghavan T (2005) Thallium: a review of public health and environmental concerns. Environ Int 31:493–501
Ralph L, Twiss M (2002) Comparative toxicity of thallium (I), thallium (III), and cadmium (II) to the unicellular alga chlorella isolated from Lake Erie. Bull Environ Contam Toxicol 68:261–268
Sabrya R, Hafeza A, Khedra M, El-Hassaninb AL (2007) Removal of lead by an emulsion liquid membrane. Desalination 212:165–175
Sato T, Sato K (1992) Liquid-liquid extraction of indium (III) from aqueous acid solutions by acid oroganophosphorus compounds. Hydrometall 30:367–383
Shirazian S, Fadaei F, Ashrafizadeh SN (2012) Modeling of thallium extraction in a hollow-fiber membrane contactor. Solvent Extr Ion Exch 30:490–506
Speller SC (2003) Thallium based high temperature superconductors for microwave device applications. Mater Sci Technol 19:269–282
Xiao T, Guha J, Boyle D, Liu C, Chen J (2004) Environmental concerns related tohigh thallium levels in soils and thallium uptake by plants in southwest Guizhou, China. Sci Total Environ 318:223–244
Zeng LL, Zhang Y, Bukirwa C, Li WS, Yang YQ (2015) Study of mean diameter and drop size distribution of emulsion drops in a modified rotating disc contactor for an emulsion liquid membrane system. RSC Adv 5:89959–89970
Zeng LL, Wang WY, Chen W, Bukirwa C, Yang YQ (2016a) Experimental and modeling of nickel removal from sulfate solutions by emulsion liquid membrane using PC 88A. Desalin Water Treat 57:11184–11194
Zeng LL, Zhang Y, Liu Q, Yang L, Xiao JP, Liu X, Yang YQ (2016b) Determination of mass transfer coefficient for continuous removal of cadmium by emulsion liquid membrane in a modified rotating disc contactor. Chem Eng J 289:452–462
Zhang L, Huang T, Zhang M, Guo X, Yuan Z (2008) Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J Hazard Mater 157:352–357
Zhang L, Huang T, Liu N, Liu X, Li H (2009) Sorption of thallium (III) ions from aqueous solutions using titanium dioxide nanoparticles. Microchim Acta 165:73–78
Zitko V (1975) Toxicity and pollution potential of thallium. Sci Total Environ 4:185–192
Acknowledgements
This research was supported by the National Science and Technology Major Project of China on the Water Pollution Control and Treatment (No. 2010ZX07212-008), the Project of Hunan Provincial Natural Science Foundation of China (No. 14JJ5027), and the industrial partner of Hunan Valin Energy-Saving & Environmental Protection Technology Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Highlights
• Industrial desulfurization effluent contained thallium was removed by an ELM system.
• Influences of different variables on thallium removal were investigated.
• The efficient treatment conditions of low concentration thallium in the ELM were optimized.
• The maximum removal efficiency of Tl can be reached nearly to 100%.
Rights and permissions
About this article
Cite this article
Yang, L., Xiao, J., Shen, Y. et al. The efficient removal of thallium from sintering flue gas desulfurization wastewater in ferrous metallurgy using emulsion liquid membrane. Environ Sci Pollut Res 24, 24214–24222 (2017). https://doi.org/10.1007/s11356-017-0040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0040-0