Abstract
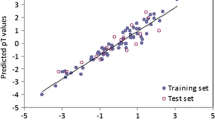
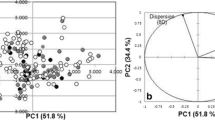
Predictive toxicology using chemometric tools can be very useful in order to fill the data gaps for ionic liquids (ILs) with limited available experimental toxicity information, in view of their growing industrial uses. Though originally promoted as green chemicals, ILs have now been shown to possess considerable toxicity against different ecological endpoints. Against this background, quantitative structure-activity relationship (QSAR) models have been developed here for the toxicity of ILs against the green algae Scenedesmus vacuolatus using computed descriptors with definite physicochemical meaning. The final models emerged from E-state indices, extended topochemical atom (ETA) indices and quantum topological molecular similarity (QTMS) indices. The developed partial least squares models support the established mechanism of toxicity of ionic liquids in terms of a surfactant action of cations and chaotropic action of anions. The models have been developed within the guidelines of the Organization of Economic Co-operation and Development (OECD) for regulatory QSAR models, and they have been validated both internally and externally using multiple strategies and also tested for applicability domain. A preliminary attempt has also been made, for the first time, to develop interspecies quantitative toxicity-toxicity relationship (QTTR) models for the algal toxicity of ILs with Daphnia toxicity, which should be interesting while predicting toxicity of ILs for an endpoint when the data for the other are available.



Similar content being viewed by others
References
Benedetti PGD, Fanelli F (2010) Computational quantum chemistry and adaptive ligand modeling in mechanistic QSAR. Drug Discov Today 15:859–866
Benigni R, Giuliani A (2003) Putting the predictive toxicology challenge into perspective: reflections on the results. Bioinformatics 19:1194–1200
Bhhatarai B, Garg R, Gramatica P (2010) Are mechanistic and statistical QSAR approaches really different? MLR studies on 158 cycloalkyl-pyranones. Mol Inf 29:511–522
Bubalo MC, Radošević K, Redovniković IR, Halambek J, Srček VG (2014) A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol Environ Saf 99:1–12
Cerius2 (2005) Cerius2 version 4.10, Accelrys Inc. San Diego, CA, USA. http://www.accelrys.com. Accessed 22 Apr 2014
Das RN, Roy K (2012) Development of classification and regression models for Vibrio fischeri toxicity of ionic liquids: green solvents for the future. Toxicol Res 1:186–195
Das RN, Roy K (2013a) Advances in QSPR/QSTR models of ionic liquids for the design of greener solvents of the future. Mol Divers 17:151–196
Das RN, Roy K (2013b) QSTR with extended topochemical atom (ETA) indices. 16. Development of predictive classification and regression models for toxicity of ionic liquids towards Daphnia magna. J Hazard Mater 166–178:254–255
Das RN, Roy K (2014) Predictive modeling studies for the ecotoxicity of ionic liquids towards the green algae Scenedesmus vacuolatus. Chemosphere 104:170–176
Fatemi MH, Izadiyan P (2011) Cytotoxicity estimation of ionic liquids based on their effective structural features. Chemosphere 84:553–563
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian Inc, Wallingford
GaussView4.1, Semichem Inc., Gaussian Inc., Pittsburgh, PA, USA, 2003
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26:694–701
Hall LH, Kier LB (2000) The E-state as the basis for molecular structure space definition and structure similarity. J Chem Inf Comput Sci 40:784–791
Irabien A, Garea A, Luis P (2009) Hybrid molecular QSAR model for toxicity estimation: application to ionic liquids. Comput Aided Chem Eng 26:63–67
Ismail Hossain M, Samir BB, El-Harbawi M, Masri AN, Abdul Mutalib MI, Hefter G, Yin C-Y (2011) Development of a novel mathematical model using a group contribution method for prediction of ionic liquid toxicities. Chemosphere 85:990–994
Izadiyan P, Fatemi MH, Izadiyan M (2013) Elicitation of the most important structural properties of ionic liquids affecting ecotoxicity in limnic green algae; a QSAR approach. Ecotoxicol Environ Saf 87:42–48
Kar S, Roy K (2010a) First report on interspecies quantitative correlation of ecotoxicity of pharmaceuticals. Chemosphere 81:738–747
Kar S, Roy K (2010b) Predictive toxicology using QSAR: a perspective. J Indian Chem Soc 87:1455–1515
Luis P, Ortiz I, Aldaco R, Irabien A (2007) A novel group contribution method in the development of a QSAR for predicting the toxicity (Vibrio fischeri EC50) of ionic liquids. Ecotoxicol Environ Saf 67:423–429
Mester P, Wagner M, Rossmanith P (2012) Ionic liquids designed as chaotrope and surfactant for use in protein chemistry. Sep Purif Technol 97:211–215
Minitab Inc. (2004) MINITAB version 14.13, Minitab Inc., USA, http://www.minitab.com/en-US/default.aspx. Accessed 22 April 2014
Mitra I, Saha A, Roy K (2010) Exploring quantitative structure-activity relationship (QSAR) studies of antioxidant phenolic compounds obtained from traditional Chinese medicinal plants. Mol Simul 36:1067–1079
O’Brien SE, Popelier PLA (2001) Quantum molecular similarity. 3. QTMS descriptors. J Chem Inf Comput Sci 41:764–775
O'Brien SE, Popelier PLA (2002) Quantum topological molecular similarity. Part 4. A QSAR study of cell growth inhibitory properties of substituted (E)-1-phenylbut-1-en-3-ones. J Chem Soc Perkin Trans 2:478–483
Popelier PLA (1996) MORPHY, a program for an automated “atoms in molecules” analysis. Comput Phys Commun 93:212–240
Popelier PLA (1999) Quantum molecular similarity. 1. BCP space. J Phys Chem A 103:2883–2890
Popelier PLA, Chaudry UA, Smith PJ (2002) Quantum topological molecular similarity. Part 5. Further development with an application to the toxicity of polychlorinated dibenzo-p-dioxins (PCDDs). J Chem Soc Perkin Trans 2:1231–1237
Rogers D (1996) Some theory and examples of genetic function approximation with comparison to evolutionary techniques. In: Devillers J (ed) Genetic algorithm in molecular modeling. Academic, London, pp 87–107
Rogers D, Hopfinger AJ (1994) Application of genetic function approximation to quantitative structure-activity relationships and quantitative structure–property relationships. J Chem Inf Comput Sci 34:854–866
Roy K, Das RN (2011) On some novel extended topochemical atom (ETA) parameters for effective encoding of chemical information and modelling of fundamental physicochemical properties. SAR QSAR Environ Res 22:451–472
Roy K, Ghosh G (2004) QSTR with extended topochemical atom indices. 2. Fish toxicity of substituted benzenes. J Chem Inf Comput Sci 44:559–567
Roy K, Mitra I (2011) On various metrics used for validation of predictive QSAR models with applications in virtual screening and focused library design. Comb Chem High Throughput Screen 14:450–474
Roy K, Mitra I, Kar S, Ojha PK, Das RN, Kabir H (2012) Comparative studies on some metrics for external validation of QSPR models. J Chem Inf Model 52:396–408
Roy K, Chakraborty P, Mitra I, Ojha PK, Kar S, Das RN (2013) Some case studies on application of “r m 2” metrics for judging quality of QSAR predictions: emphasis on scaling of response data. J Comput Chem 34:1071–1082
Roy K, Das RN, Popelier P (2014) Quantitative structure-activity relationship for toxicity of ionic liquids to Daphnia magna: aromaticity vs. lipophilicity. Chemosphere (accepted)
Smirnova NA, Safonova EA (2010) Ionic liquids as surfactants. Russ J Phys Chem A 84:1695–1704
Steudte S, Stepnowski P, Cho C-W, Thӧming J, Stolte S (2012) (Eco)toxicity of fluoro-organic and cyano-based ionic liquid anions. Chem Commun 48:9382–9384
Torrecilla JS, Palomar J, Lemus J, Rodríguez F (2010) A quantum-chemical-based guide to analyze/quantify the cytotoxicity of ionic liquids. Green Chem 12:123–134
UFT/Merck Ionic Liquids Biological Effects Database (2013) http://www.il-eco.uft.uni-bremen.de/. Accessed 22 Apr 2014
Wang R, Fu Y, Lai L (1997) A new atom-additive method for calculating partition coefficients. J Chem Inf Comput Sci 37:615–621
Williams ES, Panko J, Paustenbach DJ (2009) The European Union’s REACH regulation: a review of its history and requirements. Crit Rev Toxicol 39:553–675
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst 58:109–130
Yap CW (2011) PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32:1466–1474
Acknowledgments
KR thanks the European Commission for a Marie Curie International Incoming Fellowship (ECZ IONTOX). RND thanks CSIR, New Delhi, for a Senior Research Fellowship.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Michael Matthies
Rights and permissions
About this article
Cite this article
Roy, K., Das, R.N. & Popelier, P.L.A. Predictive QSAR modelling of algal toxicity of ionic liquids and its interspecies correlation with Daphnia toxicity. Environ Sci Pollut Res 22, 6634–6641 (2015). https://doi.org/10.1007/s11356-014-3845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3845-0