Abstract
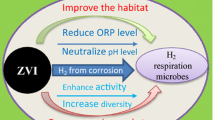
The zero-valent iron (ZVI) corrosion products and their functions were investigated in the combined ZVI and anaerobic sludge system. Results showed that ZVI corrosion occurred, and the reductive transformation and dechlorination of p-chloronitrobenzene (p-ClNB) by the anaerobic sludge were enhanced. In the combined systems with different types of ZVIs and mass ratios of anaerobic sludge to ZVI, a considerable amount of suspended iron compounds was produced and coated onto the microbial cells. However, the microbial cellular structure was damaged, and the p-ClNB reductive transformation was affected adversely after the long-term presence of nanoscale ZVI (NZVI) or reduced ZVI (RZVI) with a high concentration of 5 g L−1. The oxidized products of FeOOH and Fe3O4 were found on the surface of ZVI, which are speculated to act as electron mediators and consequently facilitate the utilization of electron donors by the anaerobic microbes.






Similar content being viewed by others
References
Agrawal A, Tratnyek PG (1996) Reduction of nitro aromatic compounds by zero-valent iron metal. Environ Sci Technol 30:153–160
American Public Health Association (1998) Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation, Washington
Aulenta F, Catervi A, Majone M, Panero S, Reale P, Rossetti S (2007) Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ Sci Technol 41:2554–2559
Chang DY, Chen TH, Liu HB, Xi YF, Qing CS, Xie QQ, Frost RL (2014) A new approach to prepare ZVI and its application in removal of Cr(VI) from aqueous solution. Chem Eng J 244:264–272
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–125
Diao M, Yao M (2009) Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene: use of zero-valent iron nanoparticles in inactivating microbes. Water Res 43:5243–5251
Gandhi S, Oh BT, Schnoor JL, Alvarez PJJ (2002) Degradation of TCE, Cr(VI), sulfate, and nitrate mixtures by granular iron in flow-through columns under different microbial conditions. Water Res 36:1973–1982
Gerlach R, Cunningham AB, Caccavo F (2000) Dissimilatory iron-reducing bacteria can influence the reduction of carbon tetrachloride by iron metal. Environ Sci Technol 34:2461–2464
Haderlein SB, Schwarzenbach RP (1995) Environmental processes influencing the rate of abiotic reduction of nitroaromatic compounds in the subsurface. In: Biodegradation of Nitroaromatic Compounds. Spain J, New York, pp. 199–225
Hartter DR (1985) The use and importance of nitroaromatic chemicals in the chemical industry. Toxicity of nitroaromatic compounds. Hemisphere, Washington, pp 1–13
He N, Li PJ, Zhou YC, Fan SX, Ren WX (2009) Degradation of pentachlorobiphenyl by a sequential treatment using Pd coated iron and an aerobic bacterium (H1). Chemosphere 76:1491–1497
Huang YH, Zhang TC (2005) Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+. Water Res 39:1751–1760
Karri S, Sierra-Alvarez R, Field JA (2005) Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnol Bioeng 92:810–819
Lee DK, Cho IC (2001) Characterization of TiO2 thin film immobilized on glass tube and its application to PCE photocatalytic destruction. Microchem J 68:215–223
Liang QQ, Zhao DY (2014) Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. J Hazard Mater 271:16–23
Liu CC, Tseng DH, Wang CY (2006) Effects of ferrous ions on the reductive dechlorination of trichloroethylene by zero-valent iron. J Hazard Mater 136:706–713
Liu HB, Chen TH, Chang DY, Chen D, Liu Y, He HP, Yuan P, Frost RL (2012) Nitrate reduction over nanoscale zero-valent iron prepared by hydrogen reduction of goethite. Mater Chem Phys 133:205–211
Ma LM, Zhang WX (2008) Enhanced biological treatment of industrial wastewater with bimetallic zero-valent iron. Environ Sci Technol 42:5384–5389
Mantha R, Taylor KE, Biswas N, Bewtra JK (2001) A continuous system for Fe0 reduction of nitrobenzene in synthetic wastewater. Environ Sci Technol 35:3231–3236
Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28:2045–2053
Min JE, Park IS, Ko S, Shin WS, Park JW (2009) Effect of phosphate and sediment bacteria on trichloroethylene dechlorination with zero valent iron. J Environ Sci Health A 44:362–369
Olegario J, Yee N, Miller M, Sczepaniak J, Manning B (2010) Reduction of Se(VI) to Se(−II) by zerovalent iron nanoparticle suspensions. J Nanoparticle Res 12:2057–2068
Perkins PS, Komisar SJ, Puhakka JA, Ferguson JF (1994) Effects of electron-donors and inhibitors on reductive decholorination of 2,4,6-trichlorophenol. Water Res 28:2101–2107
Rosenthal H, Adrian L, Steiof M (2004) Dechlorination of PCE in the presence of Fe-0 enhanced by a mixed culture containing two Dehalococcoides strains. Chemosphere 55:661–669
Ryu A, Jeong SW, Jang A, Choi H (2011) Reduction of highly concentrated nitrate using nanoscale zero-valent iron: effects of aggregation and catalyst on reactivity. Appl Catal B Environ 105:128–135
Shea PJ, Machacek TA, Comfort SD (2004) Accelerated remediation of pesticide-contaminated soil with zerovalent iron. Environ Pollut 132:183–188
Shirin S, Balakrishnan VK (2011) Using chemical reactivity to provide insights into environmental transformations of priority organic substances: the Fe-0-mediated reduction of Acid Blue 129. Environ Sci Technol 45:10369–10377
Stieber M, Putschew A, Jekel M (2011) Treatment of pharmaceuticals and diagnostic agents using zero-valent iron—kinetic studies and assessment of transformation products assay. Environ Sci Technol 45(11):4944–4950
USEPA (1988) In: Agency EP (ed) National pollutant discharge elimination system, code of federal regulations. US Government Printing Office, Washington
Velimirovic M, Simons Q, Bastiaens L (2014) Guar gum coupled microscale ZVI for in situ treatment of CAHs: continuous-flow column study. J Hazard Mater 265:20–29
Vlyssides A, Barampouti EM, Mai S (2009) Influence of ferrous iron on the granularity of a UASB reactor. Chem Eng J 146:49–56
Wang SM, Tseng SK (2009) Dechlorination of trichloroethylene by immobilized autotrophic hydrogen-bacteria and zero-valent iron. J Biosci Bioeng 107:287–292
Watanabe M, Yoneda M, Morohashi A, Hori Y, Okamoto D, Sato A, Kurioka D, Nittami T, Hirokawa Y, Shiraishi T, Kawai K, Kasai H, Totsuka Y (2013) Effects of Fe3O4 magnetic nanoparticles on A549 cells. Int J Mol Sci 14:15546–15560
Xiu ZM, Jin ZH, Long TL, Mahendra S, Lowry GV, Alvarez PJJ (2010) Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene. Bioresour Technol 101:1141–1146
Xu XH, Wo JJ, Zhang JH, Wu YJ, Liu Y (2009) Catalytic dechlorination of p-NCB in water by nanoscale Ni/Fe. Desalination 242:346–354
Zhang W, Chen L, Chen H, Xia SQ (2007) The effect of Fe-0/Fe2+/Fe3+ on nitrobenzene degradation in the anaerobic sludge. J Hazard Mater 143:57–64
Zhang YB, Jing YW, Zhang JX, Sun LF, Quan X (2011) Performance of a ZVI-UASB reactor for azo dye wastewater treatment. J Chem Technol Biotechnol 86:199–204
Zhu L, Lin HZ, Qi JQ, Xu XY, Qi HY (2012) Effect of H2 on reductive transformation of p-ClNB in a combined ZVI-anaerobic sludge system. Water Res 46:6291–6299
Acknowledgments
The works were funded by the National Natural Science Foundation of China (Nos. 50778158 and 51378454) and National Key Technologies Research and Development Program of China (No. 2013BAC16B04).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Zhu, L., Gao, K., Jin, J. et al. Analysis of ZVI corrosion products and their functions in the combined ZVI and anaerobic sludge system. Environ Sci Pollut Res 21, 12747–12756 (2014). https://doi.org/10.1007/s11356-014-3215-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3215-y