Abstract
The aim of this study was to evaluate the toxicological responses of earthworm (Eisenia fetida) induced by field-contaminated, metal-polluted soils. Biochemical responses and DNA damage of earthworm exposed to two multi-metal-contaminated soils in a steel industry park and a natural reference soil in Zijin Mountain for 2, 7, 14, and 28 days were studied. Results showed that three enzyme activities, including superoxide dismutase (SOD), acetylcholinesterase (AChE), and cellulase, in earthworm in metal-contaminated soils were significantly different from those of the reference soil. Cellulase and AChE were more sensitive than SOD to soil contamination. The Olive tail moment of the comet assay after 2-day exposure increased 56.5 and 552.0 % in two contaminated soils, respectively, compared to the reference soil. Our findings show that cellulase and DNA damage levels can be used as potential biomarkers for exposure of earthworm to metal-polluted soils.
Similar content being viewed by others
Introduction
Heavy metal contamination has become a serious problem in the environment. Due to the non-biodegradable property of heavy metals in soils, their release to the environment should be restricted (Novais et al. 2011; Maity et al. 2008). In China, heavy metal pollution in urban soils, urban road dusts, and agricultural soils became serious with the rapid industrialization and urbanization during the last two decades (Wei and Yang 2010), and the potential public health risk associated with heavy metal contamination is of current concern.
Routine chemical analyses of metals cannot take into account issues such as mixture toxicity and the environmental conditions (such as soil structure and chemical absorption, temperature, pH, etc.) determining chemical bioavailability (Gastaldi et al. 2007). For the purpose of an adequate assessment of soil pollution, the use of bioassays to rapidly screen exposure and to demonstrate potential effects of pollutants can provide an ideal means to determine the complex effects of chemical mixtures (Xiao et al. 2006c). Among soil organisms, earthworms play an important role in the soil ecosystem and appear to be one of the best organisms for use in soil toxicity evaluation. Thus, they are included in a group of key indicators for ecotoxicological testing of industrial chemicals determined by the Organisation for Economic Co-operation and Development and the European Economic Community (EEC) (Capowiez et al. 2003). The use of molecular biomarkers can be a complementary approach to standard toxicity tests (mortality and reproduction rates) because it provides more information about the organism's stress response to individual toxicants and mixtures (Gastaldi et al. 2007; Calisi et al. 2011; Hankard et al. 2004; Svendsen et al. 2004).
The activities of certain enzymes in earthworms are regarded as fast and prognostic indices of individual reaction to the environmental stress (Łaszczyca et al. 2004). Superoxide dismutase (SOD), one of the antioxidant enzymes, has been considered a good molecular bioindicator for contaminant-mediated oxidative stress to reflect the magnitude of responses in earthworms exposed to toxic metals and other xenobiotics (Novais et al. 2011; Xie et al. 2011). It has been reported that the activity of acetylcholinesterase (AChE) can be a promising parameter in evaluating the toxicity of heavy metals and pesticides (Chakra Reddy and Venkateswara Rao 2008; Ribera et al. 2001; Saint-Denis et al. 2001). Cellulase is another important enzyme in earthworms, and changes in its activity can directly influence the earthworm’s ability to decompose plant litter and other cellulosic materials (Luo et al. 2009; Shi et al. 2007).
The comet assay has become one of the standard methods for assessing DNA damage because of its simplicity, sensitivity, versatility, speed, and economy (Collins 2004). The comet assay applied to earthworms has been widely used to evaluate the genotoxicity of PAHs (Di Marzio et al. 2005), pesticides (Xiao et al. 2006a), multi-contaminated field soils (Bonnard et al. 2010; Qiao et al. 2007), and metals (Bigorgne et al. 2010; Li et al. 2009; Manerikar et al. 2008).
Many studies focus on the impact of metals in soil on earthworms, but most of these studies are performed in artificial soils or soils artificially contaminated by the addition of metal in solution, generally as a single metallic element (Nahmani et al. 2007b). Relatively few studies have dealt with the impact of field-contaminated soils with multiple contaminants (Alvarenga et al. 2008; Nahmani et al. 2007a; Berthelot et al. 2008; Bonnard et al. 2009). The present study aimed to evaluate biochemical responses and genotoxicity of earthworm (Eisenia fetida) exposed to two multi-metal-contaminated soils following long-term exposure in view of the identification of potential biomarkers for early warning of environmental health impacts. We investigated biochemical responses as measured by activities of SOD, AChE, and cellulase in earthworm, while the comet assay was applied to evaluate the genotoxicity of multi-contaminated field soils. To our knowledge, our study is the first attempt in integrating SOD, AChE, cellulase, and DNA damage to determine the effects of soil heavy metal contamination on earthworms.
Materials and methods
Experimental animal
E. fetida, one of the epigeic worm species, was adopted as the test species because it is widely available and easily reared in laboratory culture. Earthworms were obtained from a local market in Nanjing, China. Healthy adult earthworms weighing 200–300 mg and having a well-developed clitellum were used for all experiments. They were acclimatized for at least 1 week under laboratory conditions in culture pots. One hundred grams dairy manure which was dried at 100 °C and ground to pass at 2-mm sieve was incorporated into soil as food during the acclimation period. Prior to exposure, the earthworms were extracted from the culture media and placed in petri dishes for 24 h on moist filter paper at 20 ± 1 °C in the dark to void their gut contents. Petri dishes were closed using lids with air holes.
Exposure procedure
The metal-contaminated soil samples were collected from the surface layer (0–20 cm) of a steel industry park in Nanjing, China. Topsoil (0–20 cm) from an uncultivated and unpolluted field in Zijin Mountain (a mountain located in Nanjing) was used as reference soil. Before E. fetida exposure, the soil was air-dried and sieved though a 2-mm mesh net. The soil samples were subjected to chemical characterization and total main heavy metal quantification (Cd, Cr, Cu, Ni, Pb, and Zn) by inductively coupled plasma-atomic emission spectrometry (OPTIMA 5300 DV, Perkin-Elmer, USA) after digestion. The main characteristics and total metal content of the soil samples are presented in Table 1.
The experiments were conducted in wide-mouth bottles (1 L) with 700 g dry soil, and the soil moisture content was adjusted to 35 % of the water-holding capacity (WHC) with deionized water. E. fetida were introduced in bottles, and the experiments ran at 20 ± 1 °C with 16:8 light/dark photoperiod for 28 days. Each treatment contained 3 replicates, and each bottle kept 12 earthworms. The bottles were covered with plastic film that had been punched with small holes. Water was sprayed into the room of the bottle regularly to keep the air humidity at 80 % during the experimental period. No food was added to the bottles throughout the period of exposure. Three earthworms were collected from each replicate bottle on the 2nd, 7th, 14th, and 28th day for analytical procedure. No mortality was observed throughout the experimental period.
Analytical procedure
Prior to biochemical assays, each gut-cleaned earthworm (three for each group) was cut into pieces and mixed with ice-cold 0.85 % NaCl at 1/9 w/v ratio individually. The mixture was homogenized using XO-150 ultrasonic cell disrupter system (Xian’ou Instrument Corp., Nanjing, China) and then centrifuged at 3,000 rpm for 10 min at 4 °C. The resulting supernatants were used in the determination of SOD, AChE, and cellulase levels.
SOD activity was assayed by its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT), as described by Dhindsa et al. (1981). One unit of enzyme activity was defined as the amount of the enzyme exhibiting 50 % inhibition of the auto-oxidation rate of 0.1 mM NBT in a 1 mL solution at 25 °C (U/mg protein). AChE activity was spectrophotometrically determined according to Ellman (1961) by measuring the increase in absorbance of the sample at 412 nm in the presence of 1 mM acetylthiocholine as substrate and 0.1 mM 5,5-dithiobis-2-dinitrobenzoic acid. AChE activity was expressed as nanomoles of product developed per milligram of proteins per minute (nmol/min mg protein). Cellulase activity was determined according to Zhang (1991). A small volume (0.5 mL) of enzyme preparation was added into a test tube containing 1.0 mL preheated sodium carboxyl methyl cellulose, and the mixture was incubated at 50 °C for 30 min. The concentration of glucose was determined by adding 3, 5-dinitrosalicylic acid and measured spectrophotometrically (WFZ UV-2000, Unico Instrument Corp., China) at 530 nm. Enzyme activities were expressed in milligram of glucose per milligram of protein per hour (U/h mg protein). Protein content was measured according to Bradford (1976).
For the comet assay, three earthworms were used for each group after exposure. Their coelomocytes were obtained according to the non-invasive extrusion method described by Eyambe et al. (1991). Individual earthworm was initially rinsed in the extrusion medium, which consisted of 5 % ethanol, 95 % saline, 2.5 mg/mL Na2-EDTA, and 10 mg/mL guaiacol glyceryl ether (pH 7.3) to spontaneously secrete the coelomocytes in the medium. Then, the obtained coelomocytes were washed twice with phosphate-buffered saline. The cells were collected by centrifugation (3,000 rpm, 3 min) and kept at 4 °C before analysis using the comet assay. The comet assay was performed as described by Singh et al. (1988), Tice et al. (2000), and Hu et al. (2010) with some modifications. Ethidium bromide-stained nuclei were examined with a fluorescent microscope (BX41, Olympus, Japan). Three slides per group were examined, and at least 50 cells were analyzed for each slide. Images were analyzed according to the method of Collins et al. (1995) using the comet assay software project (CASP 1.2.2). Although the software reports several parameters, Olive tail moment (OTM), which is the product of the tail length and fraction of total DNA in the tail, and the percentage of DNA in the comet tail (tail DNA%) are two of the most commonly used parameters. Tail DNA% has a better linearity with dose of damage over a reasonable range and is considered to be the most reliable parameter (Collins 2004). They are presented here as measures of single-strand DNA breaks/alkali-labile sites to evaluate DNA damage of E. fetida treated with different soils.
Statistical analysis
The values of biochemical responses are mean ± SD (n = 3), while the values of comet assay are mean ± SE (n = 3). Statistical significances of differences between control and treated samples were determined by the use of one-way analysis of variance (ANOVA). ANOVA was also performed for differences between times of exposure, using SPSS 17.0 statistical software, taking p < 0.05 as significance level according to the Tukey’s post hoc test.
Results
Soil properties and metal content
Table 1 presents the soil properties and metal content in all sites. The soils were neutral, and soil from site III presented a slightly lower content in organic C than the other soils. Of the metals analyzed, the detection limits for Cd, Cr, Cu, Ni, Pb, and Zn were 0.3, 0.2, 0.2, 0.9, 3, and 0.2 mg/kg, respectively. Cd levels were below detection limit in all soil samples, and the total contents for Cr, Cu, Ni, Pb, and Zn in soils from sites II and III were high and largely exceeded the contents in reference soil. Compared with site II, soil from site III had a higher content of Cu, Pb, and Zn, which were 466, 394, and 1,729 mg/kg, respectively. However, soil from site II was characterized by a higher Cr content of 289 mg/kg.
Biochemical assays
Changes of all tested enzyme activities are summarized in Table 2, and the duration of exposure had a significant effect on all the biochemical responses studied. In some cases, significant differences (p < 0.05) were also found between controls from different time points, like, for example, in SOD activity, where a significant difference was found between controls from all four times of exposure. In general, SOD activity increased with the duration of exposure at all sites, including the controls. In contrast, cellulase activity in controls decreased over time. Similarly, AChE activity in controls decreased substantially with the duration of exposure, except on day 14.
In comparison with reference site I, a significant decrease (p < 0.05) in SOD activity was observed in site III soil for 2 days after exposure. On day 7, the SOD activity in E. fetida exposed to site II and III soil increased 40.5 and 28.2 % compared to the reference soil, respectively. However, a significant inhibition (p < 0.05) of SOD activity was found at both contaminated soils on day 14 and day 28.
A significant inhibition (p < 0.05) of AChE activity was found at both contaminated soils after 2 days of exposure. After 7 days of exposure, the AChE activity at site III soil increased significantly compared to the reference soil, while the site II soil still inhibited AChE activity. On day 14, the AChE activity increased (p < 0.05) at site III soil compared to the control. Moreover, the AChE activity of treated earthworms was markedly induced by contaminated soils at site II and site III on day 28, with increased rates of 25.5 and 36.1 % compared to the control, respectively.
The contaminated soils (site II and site III) exerted a significant inhibition effect on cellulase activity during the entire exposed time (28 days) except on day 14. On day 14, a significant increase was observed at site III. But this effect was transient and inconsistent. By 28 days of exposure, the cellulase activity at site II and site III both decreased compared to the reference soil.
Comet assay
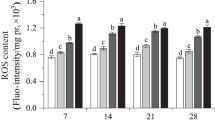
OTM, the product of the distance between the center of gravity of the head and the center of the gravity of the tail and percent tail DNA, was chosen to express the comet assay results. The results of the OTM presented in Fig. 1 show that the DNA damage of earthworm E. fetida exposed to metal-contaminated soils was always significantly higher than those exposed to reference soil through the entire experimental period. In comparison with the control, OTM of E. fetida increased 56.5 and 552.0 % on day 2 after exposure to site II and III soil, respectively. DNA damages of earthworms exposed to site III and reference soils decreased with the time of exposure during the early and middle exposed times (2, 7, and 14 days). Although the OTM of E. fetida exposed to site II soil had an elevation on day 7, all OTM, including the control, reached the lowest level on day 14. However, on day 28, there was an elevation of DNA damage in all groups after they reached the lowest level.
DNA damages of earthworm coelomocytes represented by tail DNA% are presented in Fig. 2. The results of tail DNA% were similar to the results of OTM during the exposure period. Photos of control assays show that no damage (Fig. 3a), contaminated soil exposure caused damage (Fig. 3b).
Discussion
The results demonstrate that in E. fetida, exposure to multi-metal-contaminated soils induced significant changes in all the studied biomarkers. However, some biochemical assays (SOD activity) were less sensitive to these particular types of soil contamination, whereas DNA damage and the activities of AChE and cellulase were much more sensitive.
Some reports suggest that in some cases, organisms primarily respond to certain soil attributes rather than to the pollutant concentration (Chang et al. 1997). Soil pH and organic C have been claimed to be important factors for affecting metal bioavailability to ecological receptors (Dayton et al. 2006; Peijnenburg and Jager 2003; Basta et al. 2005; Spurgeon and Hopkin 1996). In our studies, soil pH remained in the neutral to slightly alkaline range (i.e., 7.55, 7.80, and 7.95 for site I, II, and III soil, respectively), and organic C values in all soils were similar. Compared with the soil environmental quality standards (Table 3), the metal contents in contaminated soils largely exceeded the maximum permissible concentration in soils defined by many countries. Therefore, the heavy metal pollution in soils accounts for the observed toxicological responses to earthworms.
Many studies have reported the metal toxicity to earthworms tested in the artificial and field soil (Neuhauser et al. 1985; Spurgeon and Hopkin 1995; Spurgeon et al. 1994). The LC50 values of Cu, Zn, Ni, and Pb reported by Neuhauser et al. (1985) were 643 (549–753) mg/kg, 662 (574–674) mg/kg, 757 (661–867) mg/kg, and 5941 (5,292–6,670) mg/kg in artificial soil, respectively. The Zn content in the soils from site II (685 mg/kg) and site III (1,729 mg/kg) exceed the LC50 value of Zn, but no mortality was observed in our study. This confirms that toxic effects of metals were less severe in field soils (Spurgeon and Hopkin 1995). As reported by many studies, Cu and Zn were considered more toxic than other metals such as Ni and Pb (Neuhauser et al. 1985; Spurgeon and Hopkin 1995); we assumed that the contamination in site III was more severe than site II. On the other hand, it should be noted that simultaneous exposure to several metals can also lead to antagonistic, not necessarily to additive or synergistic, effects, as observed by Khalil et al. (1996).
SOD activity increased significantly at 7 days in metal-contaminated soils compared with reference soil. But the inhibition observed at 14 and 28 days was somewhat unexpected. Honsi et al. (1999) concluded that antioxidant enzymes such as SOD and catalase (CAT) in two Eisenia species were not inducible and hence not suitable as biomarkers of metal-induced oxidative stress. The results of the present study also showed that SOD is not a suitable biomarker for metal-contaminated soils in our case. In contrast, Berthelot et al. (2008) found that SOD responded well to contamination when the earthworms were exposed to metal- and energetic compound-contaminated soils in Gagetown. The toxicity mechanisms that account for these differences remain unknown. However, as Łaszczyca et al. (2004) stated that though no simple specific and unequivocal signal should be expected, activity of antioxidative enzymes (such as CAT and SOD) and glutathione S-transferase antioxidant activities are promising earthworm biomarkers of exposure in earthworms.
AChE is a critical enzyme in the nervous system of vertebrates and invertebrates and is the functional target of several xenobiotics. It is well established that AChE inhibition is a useful biomarker for organophosphate and carbamate pesticides both in in vivo and in vitro conditions (Rao and Kavitha 2004; Ribera et al. 2001; Venkateswara Rao et al. 2003; Key and Fulton 2002). However, the effect of the metal ion on the activity of AChE revealed some contradictory results as the activation observed in some studies does not coincide with the inactivation reported in other investigations (Beauvais et al. 2001; Romani et al. 2003; Frasco et al. 2005; Sarkarati et al. 1999; Zatta et al. 2002).
In our study, AChE activities in contaminated soils were inhibited after 2 days of exposure, but they increased at the 28th day of exposure. The reason for these fluctuations remains unclear. But the elevation observed on day 28 indicated an activating effect of metals on AChE and led to an improved catalytic efficiency of AChE in earthworms. Such an activating effect of metals on AChE has been described in many studies. For example, Romani et al. (2003) demonstrated an increase in AChE activity (V m/K m) after Sparus auratus was exposed to sublethal copper concentrations. Zatta et al. (2002) also found from in vitro studies that aluminum chloride had an activation of mouse brain AChE. Because of the complexity of different metal effects, it is necessary to consider the effects of metals on AChE when using this enzyme as an environmental biomarker, particularly in environments polluted with several classes of chemicals (Frasco et al. 2005).
Occurrence of cellulase in the earthworms’ gut indicates their role in the decomposition of plant litter and other cellulosic materials (Shi et al. 2007), and it has been presumed as a bioindicator for pollution by insecticides. Many pesticides (e.g., imidacloprid and acetochlor) were found to inhibit the cellulase activity of earthworms (Xiao et al. 2006b; Luo et al. 1999). Additionally, Hu et al. (2010) also found that TiO2 and ZnO nanoparticles in soil decreased the cellulase activity in earthworms. The results of our study showed that metal-contaminated soil exposure may have a negative effect on the biochemical metabolism of earthworms, thus inhibiting cellulase activity.
To explore the potential for using the biochemical responses of earthworms as biomarkers for monitoring contamination in soils, knowledge of time- and dose-dependent relationships of the responses is needed in order to use them as bioindicators (Ribera et al. 2001). Activities of SOD, AChE, and cellulase in controls were strongly affected by the duration of exposure. Such variations have been reported in previous studies (Saint-Denis et al. 1999; Saint-Denis et al. 2001; Novais et al. 2011; Shi et al. 2007). We cannot explain these variations at this moment, and further investigation is needed to examine this effect when working on biomarkers.
The comet assay is capable of examining DNA strand breaks (DSB) in individual eukaryotic cells after in vivo or in vitro exposure and is considered to be a sensitive biomarker for identification and quantification of genotoxicity (Faust 2004). Recently, the comet assay has been widely used to evaluate the genotoxicity of field soils contaminated by metals and organic pollutants (Xiao et al. 2006c; Lourenço et al. 2011). In our case, DNA integrity of earthworms was significantly affected by the exposure to the metal-contaminated soils since the damages in the DNA of coelomocytes were always significantly higher in organisms exposed to contaminated soils than in those exposed to reference soil. According to Barillet et al., some metals could induce the production and intracellular accumulation of reactive oxygen species (ROS), which yield DNA damages (Barillet et al. 2005). Many other studies have revealed the genotoxicity of heavy metals like Ni and Cr (S. A. Reinecke AJR 2004; Manerikar et al. 2008; Bigorgne et al. 2010). The constant low DSB levels in the controls are assumed to be background values derived from endogenous and unavoidable exogenous sources (Lutz 1998).
Noticeably, the DSB levels in all groups declined after the 14-day exposure, which could be attributed to either activation of antioxidant systems against ROS or activation of DNA repair mechanisms or compartmentalization of metals in different earthworm tissues leading to the reduced bioavailable metals in circulation. According to Ching et al., DNA repair systems might be activated after the invertebrate tissue has accumulated sufficient toxicant above a threshold level (Ching et al. 2001). This may explain why earthworms exposed to less contaminated soils of site II were slower in repairing DNA and still showed increased DNA damage on day 7. In contrast, earthworms exposed to heavy contaminated soils of site III recovered faster. However, Ching et al. (2001) also suggested that prolonged exposure, even at low levels, may have results in subsequent levels exceeding the threshold, thus resulting in an activation of the repair system. This is in coincidence with our results that earthworms exposed to soils of site II repaired DNA from day 7 to day 14. We speculate that elevation of DSBs levels on day 28 may be due to the lack of nutrients.
Moreover, it should be mentioned that organic pollutants may also contribute to the responses. The contaminated soils in the present study were collected from a steel industry park, and heavy metals were considered to be the critical pollutants in this area. But more extensive investigation is required to determine the interaction of the heavy metals and organic pollutants in the future.
Conclusion
The present study showed clearly that exposure to metal-contaminated soils affected SOD, AChE, and cellulase activities in earthworm E. fetida. It can be concluded that heavy metals exhibit inhibition on cellulase activity, while long-time exposure (28 days) showed an activating effect of AChE. Due to the complexity of different enzymes, further investigation is needed to explain the particular mechanisms. A clear evidence of DNA damage and repair process was found, and this indicated that metal-contaminated soils were genotoxic for E. fetida. Based on the present work, we confirmed that the comet assay is a useful technique to evaluate the toxicity of metal-contaminated soils, and DNA damages of earthworm could be used as early biomarker. As for the biochemical assay, our results showed that cellulase was more sensitive to soil contamination and it is also a promising biomarker of exposure effects.
References
Alvarenga P, Palma P, Gonçalves AP, Fernandes RM, Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of tests to assess the quality of mine-contaminated soils. Environ Geochem Hlth 30(2):95–99. doi:10.1007/s10653-008-9147-z
Barillet S, Buet A, Adam C, Devaux A (2005) Does uranium exposure induce genotoxicity in the teleostean Danio rerio? First experimental results. Radioprotection 40:175–181. doi:10.1051/radiopro:2005s1-028
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual 34(1):49–63
Beauvais SL, Jones SB, Parris JT, Brewer SK, Little EE (2001) Cholinergic and behavioral neurotoxicity of carbaryl and cadmium to larval rainbow trout (Oncorhynchus mykiss). Ecotox Environ Safe 49(1):84–90. doi:10.1006/eesa.2000.2032
Berthelot Y, Valton É, Auroy A, Trottier B, Robidoux PY (2008) Integration of toxicological and chemical tools to assess the bioavailability of metals and energetic compounds in contaminated soils. Chemosphere 74(1):166–177. doi:10.1016/j.chemosphere.2008.07.056
Bigorgne E, Cossu-Leguille C, Bonnard M, Nahmani J (2010) Genotoxic effects of nickel, trivalent and hexavalent chromium on the Eisenia fetida earthworm. Chemosphere 80(9):1109–1112. doi:10.1016/j.chemosphere.2010.05.039
Bonnard M, Devin S, Leyval C, Morel JL, Vasseur P (2010) The influence of thermal desorption on genotoxicity of multipolluted soil. Ecotox Environ Safe 73(5):955–960. doi:10.1016/j.ecoenv.2010.02.023
Bonnard M, Eom I-C, Morel J-L, Vasseur P (2009) Genotoxic and reproductive effects of an industrially contaminated soil on the earthworm Eisenia fetida. Environ Mol Mutagen 50(1):60–67. doi:10.1002/em.20436
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. doi:10.1006/abio.1976.9999
Calisi A, Lionetto MG, Schettino T (2011) Biomarker response in the earthworm Lumbricus terrestris exposed to chemical pollutants. Sci Total Environ 409(20):4456–4464. doi:10.1016/j.scitotenv.2011.06.058
Capowiez Y, Rault M, Mazzia C, Belzunces L (2003) Earthworm behaviour as a biomarker—a case study using imidacloprid. Pedobiologia 47(5–6):542–547. doi:10.1078/0031-4056-00226
Chakra Reddy N, Venkateswara Rao J (2008) Biological response of earthworm, Eisenia foetida (Savigny) to an organophosphorous pesticide, profenofos. Ecotox Environ Safe 71(2):574–582. doi:10.1016/j.ecoenv.2008.01.003
Chang LW, Meier JR, Smith MK (1997) Application of plant and earthworm bioassays to evaluate remediation of a lead-contaminated soil. Arch Environ Con Tox 32(2):166–171. doi:10.1007/s002449900170
Ching EWK, Siu WHL, Lam PKS, Xu LH, Zhang YY, Richardson BJ, Wu RSS (2001) DNA adduct formation and DNA strand breaks in green-lipped mussels (Perna viridis) exposed to benzo[a]pyrene: dose- and time-dependent relationships. Mar Pollut Bull 42(7):603–610. doi:10.1016/s0025-326x(00)00209-5
Collins AR (2004) The comet assay for DNA damage and repair principles, applications, and limitations. Mol Biotechnol 26:249–261. doi:10.1385/MB:26:3:249
Collins AR, Ma AG, Duthie SJ (1995) The kinetics of repair of oxidative DNA-damage (strand breaks and oxidized pyrimidines) in human-cells. Mutat Res-dna Repair 336(1):69–77. doi:10.1016/0921-8777(94)00043-6
Dayton EA, Basta NT, Payton ME, Bradham KD, Schroder JL, Lanno RP (2006) Evaluating the contribution of soil properties to modifying lead phytoavailability and phytotoxicity. Environ Toxicol Chem 25(3):719–725. doi:10.1897/05-307r.1
Dhindsa RS, Plumbdhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane-permeability and lipid-peroxidation, and decreased levels of superoxide-dismutase and catalase. J Exp Bot 32(126):93–101. doi:10.1093/jxb/32.1.93
Di Marzio WD, Saenz ME, Lemière S, Vasseur P (2005) Improved single-cell gel electrophoresis assay for detecting DNA damage in Eisenia foetida. Environ Mol Mutagen 46(4):246–252. doi:10.1002/em.20153
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7((2):88. doi:10.1016/0006-2952(61)90145-9
Eyambe GS, Goven AJ, Fitzpatrick LC, Venables BJ, Cooper EL (1991) A noninvasive technique for sequential collection of earthworm (Lumbricus terrestris) leukocytes during subchronic immunotoxicity studies. Lab Anim-uk 25(1):61–67. doi:10.1258/002367791780808095
Faust F (2004) The use of the alkaline comet assay with lymphocytes in human biomonitoring studies. Mutat Res-rev Mutat 566(3):209–229. doi:10.1016/j.mrrev.2003.09.007
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2005) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10(5):360–375. doi:10.1080/13547500500264660
Gastaldi L, Ranzato E, Caprì F, Hankard P, Pérès G, Canesi L, Viarengo A, Pons G (2007) Application of a biomarker battery for the evaluation of the sublethal effects of pollutants in the earthworm Eisenia andrei. Comp Biochem Phys C 146(3):398–405. doi:10.1016/j.cbpc.2007.04.014
Hankard PK, Svendsen C, Wright J, Wienberg C, Fishwick SK, Spurgeon DJ, Weeks JM (2004) Biological assessment of contaminated land using earthworm biomarkers in support of chemical analysis. Sci Total Environ 330(1–3):9–20. doi:10.1016/j.scitotenv.2003.08.023
Honsi TG, Hoel L, Stenersen JV (1999) Non-inducibility of antioxidant enzymes in the earthworms Eisenia veneta and E. fetida after exposure to heavy metals and paraquat. Pedobiologia 43(6):652–657
Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY (2010) Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 42:586–591. doi:10.1016/j.soilbio.2009.12.007
Key PB, Fulton MH (2002) Characterization of cholinesterase activity in tissues of the grass shrimp (Palaemonetes pugio). Pestic Biochem Phys 72(3):186–192. doi:10.1016/s0048-3575(02)00006-8
Khalil MA, AbdelLateif HM, Bayoumi BM, vanStraalen NM, vanGestel CAM (1996) Effects of metals and metal mixtures on survival and cocoon production of the earthworm Aporrectodea caliginosa. Pedobiologia 40(6):548–556
Łaszczyca P, Augustyniak M, Babczyńska A, Bednarska K, Kafel A, Migula P, Wilczek G, Witas I (2004) Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland). Environ Int 30(7):901–910. doi:10.1016/j.envint.2004.02.006
Li M, Liu Z, Xu Y, Cui Y, Li D, Kong Z (2009) Comparative effects of Cd and Pb on biochemical response and DNA damage in the earthworm Eisenia fetida (Annelida, Oligochaeta). Chemosphere 74(5):621–625. doi:10.1016/j.chemosphere.2008.10.048
Lourenço JI, Pereira RO, Silva AC, Morgado JM, Carvalho FP, Oliveira JM, Malta MP, Paiva AA, Mendo SA, Gonçalves FJ (2011) Genotoxic endpoints in the earthworms sub-lethal assay to evaluate natural soils contaminated by metals and radionuclides. J Hazard Mater 186(1):788–795. doi:10.1016/j.jhazmat.2010.11.073
Luo Y-R, Wang S-H, Yun M-X, Li X-Y, Wang J-J, Sun Z-J (2009) The toxic effects of ionic liquids on the activities of acetylcholinesterase and cellulase in earthworms. Chemosphere 77:313–318. doi:10.1016/j.chemosphere.2009.07.026
Luo Y, Zang Y, Zhong YA, Kong ZM (1999) Toxicological study of two novel pesticides on earthworm Eisenia foetida. Chemosphere 39(13):2347–2356. doi:10.1016/s0045-6535(99)00142-3
Lutz WK (1998) Dose–response relationships in chemical carcinogenesis: superposition of different mechanisms of action, resulting in linear–nonlinear curves, practical thresholds, J-shapes. Mutat Res-fund Mol M 405(2):117–124. doi:10.1016/s0027-5107(98)00128-6
Maity S, Roy S, Chaudhury S, Bhattacharya S (2008) Antioxidant responses of the earthworm Lampito mauritii exposed to Pb and Zn contaminated soil. Environ Pollut 151(1):1–7. doi:10.1016/j.envpol.2007.03.005
Manerikar RS, Apte AA, Ghole VS (2008) In vitro and in vivo genotoxicity assessment of Cr(VI) using comet assay in earthworm coelomocytes. Environ Toxicol Phar 25(1):63–68. doi:10.1016/j.etap.2007.08.009
Nahmani J, Hodson ME, Black S (2007a) Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils. Environ Pollut 149(1):44–58. doi:10.1016/j.envpol.2006.12.018
Nahmani J, Hodson ME, Black S (2007b) A review of studies performed to assess metal uptake by earthworms. Environ Pollut 145(2):402–424. doi:10.1016/j.envpol.2006.04.009
Neuhauser EF, Loehr RC, Milligan DL, Maleck MR (1985) Toxicity of metals to the earthworm Eisenia fetida. Biol and Fert Soils 1(3):149–152. doi:10.1007/BF00301782
Novais SC, Gomes SIL, Gravato C, Guilhermino L, De Coen W, Soares AMVM, Amorim MJB (2011) Reproduction and biochemical responses in Enchytraeus albidus (Oligochaeta) to zinc or cadmium exposures. Environ Pollut 159(7):1836–1843. doi:10.1016/j.envpol.2011.03.031
Peijnenburg W, Jager T (2003) Monitoring approaches to assess bioaccessibility and bioavailability of metals: matrix issues. Ecotox Environ Safe 56(1):63–77. doi:10.1016/s0147-6513(03)0051-4
Qiao M, Chen Y, Wang C-X, Wang Z, Zhu Y-G (2007) DNA damage and repair process in earthworm after in-vivo and in vitro exposure to soils irrigated by wastewaters. Environ Pollut 148(1):141–147. doi:10.1016/j.envpol.2006.10.033
Rao JV, Kavitha P (2004) Toxicity of azodrin on the morphology and acetylcholinesterase activity of the earthworm Eisenia foetida. Environ Res 96(3):323–327. doi:10.1016/j.envres.2004.02.014
Ribera D, Narbonne JF, Arnaud C, Saint-Denis M (2001) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil, effects of carbaryl. Soil Biol Biochem 33(7–8):1123–1130. doi:10.1016/s0038-0717(01)00035-9
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem-biol Interact 145(3):321–329. doi:10.1016/s0009-2797(03)00058-9
Reinecke SA, Reinecke AJ (2004) The comet assay as biomarker of heavy metal genotoxicity in earthworms. Arch Environ Con Tox 46:208–215. doi:10.1007/s00244-003-2253-0
Saint-Denis M, Narbonne JF, Arnaud C, Ribera D (2001) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of lead acetate. Soil Biol Biochem 33(3):395–404. doi:10.1016/s0038-0717(00)00177-2
Saint-Denis M, Narbonne JF, Arnaud C, Thybaud E, Ribera D (1999) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of benzo[a]pyrene. Soil Biol Biochem 31(13):1837–1846. doi:10.1016/s0038-0717(99)00106-6
Sarkarati B, Cokugras AN, Tezcan EF (1999) Inhibition kinetics of human serum butyrylcholinesterase by Cd2+, Zn2+ and Al3+: comparison of the effects of metal ions on cholinesterases. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol 122(2):181–190. doi:10.1016/s0742-8413(98)10102-0
Shi Y, Shi Y, Wang X, Lu Y, Yan S (2007) Comparative effects of lindane and deltamethrin on mortality, growth, and cellulase activity in earthworms (Eisenia fetida). Pestic Biochem Phys 89:31–38. doi:10.1016/j.pestbp.2007.02.005
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low-levels of DNA damage in individual cells. Exp Cell Res 175(1):184–191. doi:10.1016/0014-4827(88)90265-0
Spurgeon DJ, Hopkin SP (1995) Extrapolation of the laboratory-based OECD earthworm toxicity test to metal-contaminated field sites. Ecotoxicology 4(3):190–205. doi:10.1007/bf00116481
Spurgeon DJ, Hopkin SP (1996) Effects of variations of the organic matter content and pH of soils on the availability and toxicity of zinc to the earthworm Eisenia fetida. Pedobiologia 40(1):80–96
Spurgeon DJ, Hopkin SP, Jones DT (1994) Effects of cadmium, copper, lead and zinc on growth, reproduction and survival of the earthworm Eisenia foetida (savigny)—assessing the environmental-impact of point-source metal contamination in terrestrial ecosystems. Environ Pollut 84(2):123–130. doi:10.1016/0269-7491(94)90094-9
Svendsen C, Spurgeon DJ, Hankard PK, Weeks JM (2004) A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotox Environ Safe 57(1):20–29. doi:10.1016/j.ecoenv.2003.08.009
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35(3):206–221. doi:10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j
Venkateswara Rao J, Surya Pavan Y, Madhavendra SS (2003) Toxic effects of chlorpyrifos on morphology and acetylcholinesterase activity in the earthworm, Eisenia foetida. Ecotox Environ Safe 54(3):296–301. doi:10.1016/s0147-6513(02)00013-1
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107. doi:10.1016/j.microc.2009.09.014
Xia JC (1996) Detailed study of the soil environmental quality standards. China environmental science publisher, Beijing, China (in Chinese)
Xiao N-W, Song Y, Ge F, Liu X-H, Ou-Yang Z-Y (2006a) Biomarkers responses of the earthworm Eisenia fetida to acetochlor exposure in OECD soil. Chemosphere 65(6):907–912. doi:10.1016/j.chemosphere.2006.03.060
Xiao NW, Jing B, Ge F, Liu XH (2006b) The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere 62(8):1366–1373. doi:10.1016/j.chemosphere.2006.07.043
Xiao RY, Wang ZJ, Wang CX, Yu G, Zhu YG (2006c) Genotoxic risk identification of soil contamination at a major industrialized city in northeast China by a combination of in vitro and in vivo bioassays. Environ Sci Technol 40(19):6170–6175. doi:10.1021/es0607335
Xie X, Wu Y, Zhu M, Y-k Z, Wang X (2011) Hydroxyl radical generation and oxidative stress in earthworms (Eisenia fetida) exposed to decabromodiphenyl ether (BDE-209). Ecotoxicology 20(5):993–999. doi:10.1007/s10646-011-0645-x
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59(1):41–45. doi:10.1016/s0361-9230(02)00836-5
Zhang DA (1991) The experimental handbook of biological macromolecule. The press of Jilin University. Changchun, China (in Chinese)
Acknowledgments
This work was supported by the Science and Technology Support Program of Jiangsu Province (No. BE2012737), Public Sector Special Scientific Research Program of the National Environmental Protection Ministry (No. 2011467054), and National Natural Science Foundation of China (No. 21077052). The authors are grateful to Richard A. Manderville for his assistance and helpful advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zheng, K., Liu, Z., Li, Y. et al. Toxicological responses of earthworm (Eisenia fetida) exposed to metal-contaminated soils. Environ Sci Pollut Res 20, 8382–8390 (2013). https://doi.org/10.1007/s11356-013-1689-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1689-7