Abstract
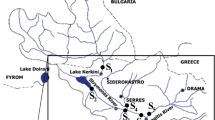
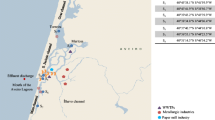
The contamination of aquatic systems by endocrine disrupting chemicals (EDCs) is now a widely established fact. Nevertheless, there is still a scarcity of knowledge concerning the source, transport, fate and bioavailability of such active compounds. In the present study we assessed the distribution of estrogenic, (anti-)androgenic, pregnane X receptor-like (PXR) and dioxin-like activities between sediment and water compartments using a polar organic compound integrative sampler (POCIS) and a semi-permeable membrane device (SPMD) passive sampler in a river where sediment has been previously described as highly and multi-contaminated. We first confirmed the contamination pattern of this river sediment between 2004, 2009 and 2010 samples, suggesting that this river is subject to a constant high contamination level. However, we showed a different distribution pattern of these activities between compartments: estrogenic activity was mainly detected in POCIS extracts and to a lesser extent in sediment and SPMD extracts; anti-androgenic activities were mainly detected in SPMD and sediment extracts while no activity was detected in POCIS extracts; PXR-like activity was detected in all three investigated compartments, with POCIS > SPMD > sediment; dioxin-like activity was mainly found in the sediment and the SPMD extracts. Overall, partitioning of the biological activities was in accordance with physicochemical properties (e.g., log K ow) of typical known active chemicals in each bioassay. Furthermore, in order to establish whether the chemicals involved in these activities were similar between the compartments, we fractionated sediment, POCIS and SPMD extracts using a multi-step fractionation procedure. This highlighted differences in the nature of active chemicals between compartments. Altogether, our results support the need to consider different compartments in order to enhance exposure assessment.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ahn YG, Shin JH, Kim HY, Khim J, Lee MK, Hong J (2007) Application of solid-phase extraction coupled with freezing-lipid filtration clean-up for the determination of endocrine-disrupting phenols in fish. Anal Chim Acta 603:67–75
Aït-Aïssa S, Laskowski S, Laville N, Porcher JM, Brion F (2010) Anti-androgenic activities of environmental pesticides in the MDA-kb2 reporter cell line. Toxicol Vitro 24:1979–1985
Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23:1640–1648
Alvarez DA, Cranor WL, Perkins SD, Clark RC, Smith SB (2008) Chemical and toxicologic assessment of organic contaminants in surface water using passive samplers. J Environ Qual 37:1024–1033
Balaguer P, Francois F, Comunale F, Fenet H, Boussioux AM, Pons M, Nicolas JC, Casellas C (1999) Reporter cell lines to study the estrogenic effects of xenoestrogens. Sci Total Environ 233:47–56
Brack W, Schirmer K, Erdinger L, Hollert H (2005) Effect-directed analysis of mutagens and ethoxyresorufin-O-deethylase inducers in aquatic sediments. Environ Toxicol Chem 24:2445–2458
Brack W, Bandow N, Schwab K, Schulze T, Streck G (2009) Bioavailability in effect-directed analysis of organic toxicants in sediments. Trac-Trends Anal Chem 28:543–549
Campbell CG, Borglin SE, Green FB, Grayson A, Wozei E, Stringfellow WT (2006) Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: a review. Chemosphere 65:1265–1280
Creusot N, Budzinski H, Balaguer P, Kinani S, Porcher JM, Aït-Aïssa S (in press) Effect-directed analysis of endocrine disrupting compounds in multi-contaminated sediment: identification of novel ligands of estrogen and pregnane X receptors. Anal Bioanal Chem
Creusot N, Kinani S, Balaguer P, Tapie N, LeMenach K, Maillot-Marechal E, Porcher JM, Budzinski H, Ait-Aissa S (2010) Evaluation of an hPXR reporter gene assay for the detection of aquatic emerging pollutants: screening of chemicals and application to water samples. Anal Bioanal Chem 396:569–583
Creusot N, Dévier M, Porcher J, Budzinski H, Aït-Aïssa S (2011a) Identification and compartmentalization study of emerging EDCs in an impacted river systems by using an EDA approach. Abstract book of the 21st European SETAC, 15–19 May 2011, Milan
Creusot N, Dévier M, Porcher J, Budzinski H, Aït-Aïssa S (2011b) Optimization of accelerated solvent extraction (ASE) of wide range of endocrine disrupting chemicals (EDCs) in sediment. Abstract book of the 21st European SETAC, 15–19 May 2011, Milan
Creusot N, Porcher J, Budzinski H, Aït-Aïssa S (2011c) Multistep-fractionation based on normal phase SPE and reverse phase HPLC (RP-HPLC) for isolation of endocrine disrupting chemicals in environmental extracts. Abstract book of the 21st European SETAC, 15–19 May 2011, Milan
David A, Gomez E, Ait-Aissa S, Bachelot M, Rosain D, Casellas C, Fenet H (2010) Monitoring organic contaminants in small French coastal lagoons: comparison of levels in mussel, passive sampler and sediment. J Environ Monit 12:1471–1481
Devier M-H, Mazellier P, Ait-Aissa S, Budzinski H (2011) New challenges in environmental analytical chemistry: identification of toxic compounds in complex mixtures. C R Chimie 14:766–779
Eggen RIL, Segner H (2003) The potential of mechanism-based bioanalytical tools in ecotoxicological exposure and effect assessment. Anal Bioanal Chem 377:386–396
EPA-3604A (1994) Method 3640A: gel-permeation clean-up
Esteve-Turrillas FA, Pastor A, Yusa V, de la Guardia M (2007) Using semi-permeable membrane devices as passive samplers. Trac-Trends Anal Chem 26:703–712
Grover DP, Balaam J, Pacitto S, Readman JW, White S, Zhou JL (2011) Endocrine disrupting activities in sewage effluent and river water determined by chemical analysis and in vitro assay in the context of granular activated carbon upgrade. Chemosphere 84:1512–1520
Harman C, Farmen E, Tollefsen KE (2010) Monitoring North Sea oil production discharges using passive sampling devices coupled with in vitro bioassay techniques. J Environ Monit 12:1699–1708
Harman C, Allan IJ, Vermeirssen E (2012) Calibration and use of the polar organic chemical integrative sampler—a critical review. Environ Toxicol Chem 31:2724–2738
Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE (2008) Fifteen years after "Wingspread" — environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci 105:235–259
Houtman CJ, Booij P, Jover E, del Rio DP, Swart K, van Velzen M, Vreuls R, Legler J, Brouwer A, Lamoree MH (2006) Estrogenic and dioxin-like compounds in sediment from Zierikzee harbour identified with CALUX assay-directed fractionation combined with one and two dimensional gas chromatography analyses. Chemosphere 65:2244–2252
ISO-8245 (1999) ISO 8245:1999: water quality — guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC). International Organization of Standardization
Jondeau-Cabaton A, Soucasse A, Jamin E, Creusot N, Grimaldi M,Jouanin I, Aït-Aïssa S, Balaguer P, Debrauwer L, Zalko D (in press) Characterization of endocrine disruptors from a complex matrix using Estrogen Receptor affinity columns and High Performance Liquid Chromatrography-High Resolution Mass Spectrometry. Environ Sci Pollut Res
Kinani S, Bouchonnet S, Creusot N, Bourcier S, Balaguer P, Porcher JM, Ait-Aissa S (2010) Bioanalytical characterisation of multiple endocrine- and dioxin-like activities in sediments from reference and impacted small rivers. Environ Pollut 158:74–83
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Lagana A, Bacaloni A, De Leva I, Faberi A, Fago G, Marino A (2004) Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal Chim Acta 501:79–88
Lemaire G, Mnif W, Pascussi JM, Pillon A, Rabenoelina F, Fenet H, Gomez E, Casellas C, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P (2006) Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol Sci 91:501–509
Leppanen MT, Kukkonen JVK (2006) Evaluating the role of desorption in bioavailability of sediment-associated contaminants using oligochaetes, semipermeable membrane devices and Tenax extraction. Environ Pollut 140:150–163
Liu R, Zhou JL, Wilding A (2004) Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction-gas chromatography–mass spectrometry. J Chromatogr A 1022:179–189
Louiz I, Kinani S, Gouze ME, Ben-Attia M, Menif D, Bouchonnet S, Porcher JM, Ben-Hassine OK, Ait-Aissa S (2008) Monitoring of dioxin-like, estrogenic and anti-androgenic activities in sediments of the Bizerta lagoon (Tunisia) by means of in vitro cell-based bioassays: contribution of low concentrations of polynuclear aromatic hydrocarbons (PAHs). Sci Total Environ 402:318–329
Mnif W, Pascussi JM, Pillon A, Escande A, Bartegi A, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P (2007) Estrogens and antiestrogens activate hPXR. Toxicol Lett 170:19–29
Mnif W, Dagnino S, Escande A, Pillon A, Fenet H, Gomez E, Casellas C, Duchesne MJ, Hernandez-Raquet G, Cavailles V, Balaguer P, Bartegi A (2010) Biological Analysis of Endocrine-disrupting compounds in Tunisian sewage treatment plants. Arch Environ Contam Toxicol 59:1–12
Petty JD, Jones SB, Huckins JN, Cranor WL, Parris JT, McTague TB, Boyle TP (2000) An approach for assessment of water quality using semipermeable membrane devices (SPMDs) and bioindicator tests. Chemosphere 41:311–321
Pojana G, Gomiero A, Jonkers N, Marcomini A (2007) Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ Int 33:929–936
Sanchez W, Ait-Aissa S, Palluel O, Ditche J-M, Porcher J-M (2007) Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasterosteus aculeatus L.) sampled in contaminated streams. Ecotoxicology 16:279–287
Schmitt C, Balaam J, Leonards P, Brix R, Streck G, Tuikka A, Bervoets L, Brack W, van Hattum B, Meire P, de Deckere E (2010) Characterizing field sediments from three European river basins with special emphasis on endocrine effects — a recommendation for Potamopyrgus antipodarum as test organism. Chemosphere 80:13–19
Tapie N, Devier MH, Soulier C, Creusot N, Le Menach K, Ait-Aissa S, Vrana B, Budzinski H (2011) Passive samplers for chemical substance monitoring and associated toxicity assessment in water. Water Sci Technol 63:2418–2426
Togola A, Budzinski H (2007) Development of polar organic integrative samplers for analysis of pharmaceuticals in aquatic systems. Anal Chem 79:6734–6741
Urbatzka R, van Cauwenberge A, Maggioni S, Viganò L, Mandich A, Benfenati E, Lutz I, Kloas W (2007) Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere 67:1080–1087
Vermeirssen ELM, Korner O, Schonenberger R, Suter MJF, Burkhardt-Holm P (2005) Characterization of environmental estrogens in river water using a three pronged approach: active and passive water sampling and the analysis of accumulated estrogens in the bile of caged fish. Environ Sci Technol 39:8191–8198
Vondracek J, Machala M, Minksova K, Blaha L, Murk AJ, Kozubik A, Hofmanova J, Hilscherova K, Ulrich R, Ciganek M, Neca J, Svrckova D, Holoubek I (2001) Monitoring river sediments contaminated predominantly with polyaromatic hydrocarbons by chemical and in vitro bioassay techniques. Environ Toxicol Chem 20:1499–1506
Vrana B (2005) Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem 24:845–868
Vrana B, Schuurmann G (2002) Calibrating the uptake kinetics of semipermeable membrane devices in water: impact of hydrodynamics. Environ Sci Technol 36:290–296
Wang L, Ying G-G, Zhao J-L, Liu S, Yang B, Zhou L-J, Tao R, Su H-C (2011) Assessing estrogenic activity in surface water and sediment of the Liao River system in northeast China using combined chemical and biological tools. Environ Pollut 159:148–156
Weiss JM, Hamers T, Thomas KV, van der Linden S, Leonards PEG, Lamoree MH (2009) Masking effect of anti-androgens on androgenic activity in European river sediment unveiled by effect-directed analysis. Anal Bioanal Chem 394:1385–1397
Wilson VS, Bobseine K, Lambright CR, Gray LE (2002) A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci 66:69–81
Xue ND, Xu XB, Jin ZL (2005) Screening 31 endocrine-disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir. Chemosphere 61:1594–1606
Ying GG, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates — a review. Environ Int 28:215–226
Zhang X, Li Q, Li G, Wang Z, Yan C (2009) Levels of estrogenic compounds in Xiamen Bay sediment, China. Mar Pollut Bull 58:1210–1216
Acknowledgements
This work was funded by grant the French Ministry of Ecology (P190-AP2010, P189-ECOPI). NC was supported by a doctoral fellowship from ANRT and INERIS. We wish to thank two anonymous reviewers for helping to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 534 kb)
Rights and permissions
About this article
Cite this article
Creusot, N., Tapie, N., Piccini, B. et al. Distribution of steroid- and dioxin-like activities between sediments, POCIS and SPMD in a French river subject to mixed pressures. Environ Sci Pollut Res 20, 2784–2794 (2013). https://doi.org/10.1007/s11356-012-1452-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1452-5