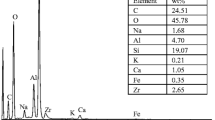
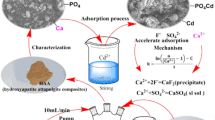
Abstract
A novel composite material, i.e., surfactant-modified hydroxyapatite/zeolite composite, was used as an adsorbent to remove humic acid (HA) and copper(II) from aqueous solution. Hydroxyapatite/zeolite composite (HZC) and surfactant-modified HZC (SMHZC) were prepared and characterized by X-ray diffraction, Fourier transform infrared spectroscopy, and field emission scanning electron microscope. The adsorption of HA and copper(II) on SMHZC was investigated. For comparison purposes, HA adsorption onto HZC was also investigated. SMHZC exhibited much higher HA adsorption capacity than HZC. The HA adsorption capacity for SMHZC decreased slightly with increasing pH from 3 to 8 but decreased significantly with increasing pH from 8 to 12. The copper(II) adsorption capacity for SMHZC increased with increasing pH from 3 to 6.5. The adsorption kinetic data of HA and copper(II) on SMHZC obeyed a pseudo-second-order kinetic model. The adsorption of HA and copper(II) on SMHZC took place in three different stages: fast external surface adsorption, gradual adsorption controlled by both film and intra-particle diffusions, and final equilibrium stage. The equilibrium adsorption data of HA on SMHZC better fitted to the Langmuir isotherm model than the Freundlich isotherm model. The equilibrium adsorption data of copper(II) on SMHZC could be described by the Langmuir, Freundlich, and Dubinin–Radushkevich isotherm models. The presence of copper(II) in solution enhanced HA adsorption onto SMHZC. The presence of HA in solution enhanced copper(II) adsorption onto SMHZC. The mechanisms for the adsorption of HA on SMHZC at pH 7 may include electrostatic attraction, organic partitioning, hydrogen bonding, and Lewis acid–base interaction. The mechanisms for the adsorption of copper(II) on SMHZC at pH 6 may include surface complexation, ion exchange, and dissolution–precipitation. The obtained results indicate that SMHZC can be used as an effective adsorbent to simultaneously remove HA and copper(II) from water.















Similar content being viewed by others
References
Balistrieri LS, Murray JW (1981) The surface chemistry of goethite (α-FeOOH) in major ion seawater. Am J Sci 281:788–806
Bia G, Pauli CPD, Borgnino L (2012) The role of Fe(III) modified montmorillonite on fluoride mobility: adsorption experiments and competition with phosphate. J Environ Manag 100:1–9
Bouhamed F, Elouear Z, Bouzid J (2012) Adsorptive removal of copper(II) from aqueous solutions on activated carbon prepared from Tunisian date stones: equilibrium, kinetics and thermodynamics. J Taiwan Inst Chem Eng. doi:10.1016/j.jtice.2012.02.011
Bouyarmane H, Asri SE, Rami A, Roux C, Mahly MA, Saoiabi A, Coradinc T, Laghzizil A (2010) Pyridine and phenol removal using natural and synthetic apatites as low cost sorbents: influence of porosity and surface interactions. J Hazard Mater 181:736–741
Chao HP, Chen SH (2012) Adsorption characteristics of both cationic and oxyanionic metal ions on hexadecyltrimethylammonium bromide-modified NaY zeolite. Chem Eng J 193–194:283–289
Chen JP, Wu SN (2004) Simultaneous adsorption of copper ions and humic acid onto an activated carbon. J Colloid Interf Sci 280:334–342
Chen H, Zhao J, Wu JY, Dai GL (2011) Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J Hazard Mater 192:246–254
Corami A, Mignardi S, Ferrini V (2007) Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. J Hazard Mater 146:164–170
Corami A, Mignardi S, Ferrini V (2008) Cadmium removal from single- and multi-metal (Cd+Pb+Zn+Cu) solutions by sorption on hydroxyapatite. J Colloid Interf Sci 317:402–408
Da’na E, Sayari A (2011) Optimization of copper removal efficiency by adsorption on amine-modified SBA-15: experimental design methodology. Chem Eng J 167:91–98
Daifullah AAM, Girgis BS, Gad HMH (2004) A study of the factors affecting the removal of humic acid by activated carbon prepared from biomass material. Colloid Surface A 235:1–10
Demirbas E, Dizge N, Sulak MT, Kobya M (2009) Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem Eng J 148:480–487
Ding CL, Shang C (2010) Mechanisms controlling adsorption of natural organic matter on surfactant-modified iron oxide-coated sand. Water Res 44:3651–3658
Doulia D, Leodopoulos C, Gimouhopoulos K, Rigas F (2009) Adsorption of humic acid on acid-activated Greek bentonite. J Colloid Interf Sci 340:131–141
Elaiopoulos K, Perraki T, Grigoropoulou E (2010) Monitoring the effect of hydrothermal treatments on the structure of a natural zeolite through a combined XRD, FTIR, XRF, SEM and N2-porosimetry analysis. Microporous Mesoporous Mater 134:29–43
Elkady MF, Mahmoud MM, Abd-El-Rahman HM (2011) Kinetic approach for cadmium sorption using microwave synthesized nano-hydroxyapatite. J Non-Cryst Solids 357:1118–1129
Engates KE, Shipley HJ (2011) Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration, and exhaustion. Environ Sci Pollut Res 18:386–395
Gasser MS, Mohsen HT, Aly HF (2008) Humic acid adsorption onto Mg/Fe layered double hydroxide. Colloid Surface A 331:195–201
Guan HD, Bestland E, Zhu CY, Zhu HL, Albertsdottir D, Hutson J, Simmons CT, Ginic-Markovic M, Tao X, Ellis AV (2010) Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J Hazard Mater 183:616–621
Hartono T, Wang SB, Ma Q, Zhu ZH (2009) Layer structured graphite oxide as a novel adsorbent for humic acid removal from aqueous solution. J Colloid Interf Sci 333:114–119
Hasret E, Ipekoglu M, Altintas S, Ipekoglu NA (2012) Microwave-assisted synthesis of hydroxyapatite for the removal of lead(II) from aqueous solutions. Environ Sci Pollut Res 19:2766–2775
Imyim A, Prapalimrungsi E (2010) Humic acids removal from water by aminopropyl functionalized rice husk ash. J Hazard Mater 184:775–781
Jamil TS, Ibrahim HS, Abd El-Maksoud IH, El-Wakeel ST (2010) Application of zeolite prepared from Egyptian kaolin for removal of heavy metals: I. Optimum conditions. Desalination 258:34–40
Jarvis KL, Majewski P (2012) Plasma polymerized allylamine coated quartz particles for humic acid removal. J Colloid Interf Sci 380:150–158
Lee SM, Laldawngliana C, Tiwari D (2012) Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem Eng J 195–196:103–111
Li ZH, Burt T, Bowman RS (2000) Sorption of ionizable organic solutes by surfactant-modified zeolite. Environ Sci Technol 34:3756–3760
Li CJ, Dong Y, Wu DY, Peng LC, Kong HN (2011) Surfactant modified zeolite as adsorbent for removal of humic acid from water. Appl Clay Sci 52:353–357
Maghsoodloo S, Noroozi B, Haghi AK, Sorial GA (2011) Consequence of chitosan treating on the adsorption of humic acid by granular activated carbon. J Hazard Mater 191:380–387
Malekian R, Abedi-Koupai J, Eslamian SS (2011) Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J Hazard Mater 185:970–976
Meski S, Ziani S, Khireddine H, Boudboub S, Zaidi S (2011) Factorial design analysis for sorption of zinc on hydroxyapatite. J Hazard Mater 186:1007–1017
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92:42–48
Motsi T, Rowson NA, Simmons MJH (2011) Kinetic studies of the removal of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 101:42–49
Moussavi G, Talebi S, Farrokhi M, Sabouti RM (2011) The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem Eng J 171:1159–1169
Neupane G, Donahoe RJ (2012) Attenuation of trace elements in coal fly ash leachates by surfactant-modified zeolite. J Hazard Mater 229–230:201–208
Rauthula MS, Srivastava VC (2011) Studies on adsorption/desorption of nitrobenzene and humic acid onto/from activated carbon. Chem Eng J 168:35–43
Shaltout AA, Allam MA, Moharram MA (2011) FTIR spectroscopic, thermal and XRD characterization of hydroxyapatite from new natural sources. Spectrochim Acta A 83:56–60
Šljivić M, Smičiklas I, Pejanović S, Plećaš I (2009a) Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl Clay Sci 43:33–40
Šljivić M, Smičiklas I, Plećaš I, Mitrić M (2009b) The influence of equilibration conditions and hydroxyapatite physico-chemical properties onto retention of Cu2+ ion. Chem Eng J 148:80–88
Smičiklas ID, Milonjić SK, Pfendt P, Raičević S (2000) The point of zero charge and sorption of cadmium (II) and strontium (II) ions on synthetic hydroxyapatite. Sep Purif Technol 18:185–194
Smičiklas I, Dimović S, Plećaš I, Mitrić M (2006) Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res 40:2267–2274
Stárek J, Zukal A, Rathouský J (1994) Comparison of the adsorption of humic acids from aqueous solutions on active carbon and activated charcoal cloths. Carbon 32:207–211
Sulaymon AH, Ebrahim SE, Mohammed-Ridha MJ (2012) Equilibrium, kinetic, and thermodynamic biosorption of Pb(II), Cr(III), and Cd(II) ions by dead anaerobic biomass from synthetic wastewater. Environ Sci Pollut Res. doi:10.1007/s11356-012-0854-8
Suresh Kumar G, Girija EK, Thamizhavel A, Yokogawa Y, Narayana Kalkura S (2010) Synthesis and characterization of bioactive hydroxyapatite-calcite nanocomposite for biomedical applications. J Colloid Interf Sci 349:56–62
Tanaka H, Watanabe T, Chikazawa M (1997) FTIR and TPD studies on the adsorption of pyridine, n-butylamine and acetic acid on calcium hydroxyapatite. J Chem Soc, Faraday Trans 93:4377–4381
Tang YL, Liang S, Yu SL, Gao NY, Zhang J, Guo HC, Wang YL (2012) Enhanced adsorption of humic acid on amine functionalized magnetic mesoporous composite microspheres. Colloid Surface A 406:61–67
Tao Q, Xu ZY, Wang JH, Liu FL, Wan HQ, Zheng SR (2010) Adsorption of humic acid to aminopropyl functionalized SBA-15. Microporous Mesoporous Mater 131:177–185
Terdkiatburana T, Wang SB, Tadé MO (2008) Competition and complexation of heavy metal ions and humic acid on zeolitic MCM-22 and activated carbon. Chem Eng J 139:437–444
Tong KS, Jain Kassim M, Azraa A (2011) Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: equilibrium, kinetics, and thermodynamic studies. Chem Eng J 170:145–153
Venkatesha TG, Viswanatha R, Arthoba Nayaka Y, Chethana BK (2012) Kinetics and thermodynamics of reactive and vat dyes adsorption on MgO nanoparticles. Chem Eng J 198–199:1–10
Wang SB, Ariyanto E (2007) Competitive adsorption of malachite green and Pb ions on natural zeolite. J Colloid Interf Sci 314:25–31
Wang SG, Gong WX, Liu XW, Gao BY, Yue QY (2006) Removal of fulvic acids using the surfactant modified zeolite in a fixed-bed reactor. Sep Purif Technol 51:367–373
Wang SB, Terdkiatburana T, Tadé MO (2008) Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems. Sep Purif Technol 62:64–70
Wang YJ, Chen JH, Cui YX, Wang SQ, Zhou DM (2009) Effects of low-molecular-weight organic acids on Cu(II) adsorption onto hydroxyapatite nanoparticles. J Hazard Mater 162:1135–1140
Wang JN, Zhou Y, Li AM, Xu L (2010) Adsorption of humic acid by bi-functional resin JN-10 and the effect of alkali-earth metal ions on the adsorption. J Hazard Mater 176:1018–1026
Wang XJ, Liang X, Wang Y, Wang X, Liu M, Yin DQ, Xia SQ, Zhao JF, Zhang YL (2011a) Adsorption of Copper (II) onto activated carbons from sewage sludge by microwave-induced phosphoric acid and zinc chloride activation. Desalination 278:231–237
Wang JH, Han XJ, Ma HR, Ji YF, Bi LJ (2011b) Adsorptive removal of humic acid from aqueous solution on polyaniline/attapulgite composite. Chem Eng J 173:171–177
Wang MS, Liao LB, Zhang XL, Li ZH (2011c) Adsorption of low concentration humic acid from water by palygorskite. Appl Clay Sci. doi:10.1016/j.clay.2011.09.012
Wei W, Zhang X, Cui J, Wei ZG (2011) Interaction between low molecular weight organic acids and hydroxyapatite. Colloid Surface A 392(1):67–75
Wen QX, Chen ZQ, Lian JX, Feng YJ, Ren NQ (2012) Removal of nitrobenzene from aqueous solution by a novel lipoid adsorption material (LAM). J Hazard Mater 209–210:226–232
Wu LM, Forsling W, Schindler PW (1991) Surface complexation of calcium minerals in aqueous solutions. 1. Surface protonation of fluoroapatite water interfaces. J Colloid Interf Sci 147:178–185
Wu YH, Wen YJ, Zhou JX, Cao JL, Jin YP, Wu YY (2012) Comparative and competitive adsorption of Cr(VI), As(III), and Ni(II) onto coconut charcoal. Environ Sci Pollut Res. doi:10.1007/s11356-012-1066-y
Yadav S, Srivastava V, Banerjee S, Gode F, Sharma YC (2012) Studies on the removal of nickel from aqueous solutions using modified riverbed sand. Environ Sci Pollut Res. doi:10.1007/s11356-012-0892-2
Zhan YH, Zhu ZL, Lin JW, Qiu YL, Zhao JF (2010) Removal of humic acid from aqueous solution by cetylpyridinium bromide modified zeolite. J Environ Sci 22:1327–1334
Zhu RH, Yu RB, Yao JX, Mao D, Xing CJ, Wang D (2008) Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catal Today 139:94–99
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50908142); the Foundation of Key Laboratory of Yangtze River Water Environment, Ministry of Education (Tongji University), China, (YRWEF201107); the Scientific Research Project of Shanghai Science and Technology Committee (10230502900); the Startup Foundation for Doctors of Shanghai Ocean University (B-5301-11-0219); and the Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50702). We also thank the editors and the anonymous reviewers whose comments and suggestions greatly improved the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhan, Y., Lin, J. & Li, J. Preparation and characterization of surfactant-modified hydroxyapatite/zeolite composite and its adsorption behavior toward humic acid and copper(II). Environ Sci Pollut Res 20, 2512–2526 (2013). https://doi.org/10.1007/s11356-012-1136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1136-1