Abstract
Introduction
The removal of heavy metals by natural adsorbent has become one of the most attractive solutions for environmental remediation. Natural clay collected from the Late Cretaceous Aleg formation, Tunisia was used as a natural adsorbent for the removal of Hg(II) in aqueous system.
Methods
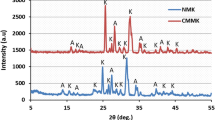
Physicochemical characterization of the adsorbent was carried out with the aid of various techniques, including chemical analysis, X-ray diffraction, Fourier transform infrared and scanning electron micrograph. Batch sorption technique was selected as an appropriate technique in the current study. Method parameters, including pH, temperature, initial metal concentration and contact time, were varied in order to quantitatively evaluate their effects on Hg(II) adsorption onto the original and pillared clay samples. Adsorption kinetic was studied by fitting the experimental results to the pseudo-first-order and pseudo-second-order kinetic models. The adsorption data were also simulated with Langmuir, Freundlich and Temkin isotherms.
Results
Results showed that the natural clay samples are mainly composed of silica, alumina, iron, calcium and magnesium oxides. The sorbents are mainly mesoporous materials with specific surface area of <250 m2 g−1. From the adsorption of Hg(II) studies, experimental data demonstrated a high degree of fitness to the pseudo-second-order kinetics with an equilibration time of 240 min. The equilibrium data showed the best model fit to Langmuir model with the maximum adsorption capacities of 9.70 and 49.75 mg g−1 for the original and aluminium pillared clays, respectively. The maximum adsorption of Hg(II) on the aluminium pillared clay was observed to occur at pH 3.2. The calculated thermodynamic parameters (∆G°, ∆H° and ∆S°) showed an exothermic adsorption process. The entropy values varied between 60.77 and 117.59 J mol−1 K−1, and those of enthalpy ranged from 16.31 to 30.77 kJ mol−1. The equilibrium parameter (R L) indicated that the adsorption of Hg(II) on Tunisian smectitic clays was favourable under the experimental conditions of this study.
Conclusion
The clay of the Aleg formation, Tunisia was found to be an efficient adsorbent for Hg(II) removal in aqueous systems.







Similar content being viewed by others
References
Akcay M (2004) Characterization and determination of the thermodynamic and kinetic properties of p-CP adsorption onto organophilic bentonite from aqueous solution. J Colloid Interface Sci 208:299–304
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Bayramoğlu G, Arica MY (2007) Kinetics of mercury ions removal from synthetic aqueous solutions using by novel magnetic p(GMA-MMA-EGDMA) beads. J Hazard Mater 144:449–457
Benzina M (1990) Contribution à l’étude cinétique et thermodynamique de l’adsorption des vapeurs organiques sur des argiles locales, Modélisation d’un adsorbeur à lit fixe. Thèse de Doctorat es-science physique, Faculté des Sciences de Tunis, p. 25
Bergaya F (1995) The meaning surface area and porosity measurements of clays and pillared clays. J Porous Mater 2:91–96
Bhakta JN, Salim M, Yamasaki K, Munekage Y (2009) Mercury adsorption stoichiometry of ceramic and activated carbon from aqueous phase under different pH and temperature. ARPN J Eng Appl Sci 4:52–59
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140:114–131
Clarkson TW (1993) Mercury: major issues in environmental health. Environ Health Perspect 100:31–38
Eloussaief M, Benzina M (2010) Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions. J Hazard Mater 178:753–757
Eloussaief M, Jarraya I, Benzina M (2009) Adsorption of copper ions on two clays from Tunisia: pH and temperature effects. Appl Clay Sci 46:409–413
Eloussaief M, Kallel N, Yaacoubi A, Benzina M (2011) Mineralogical identification, spectroscopic characterization, and potential environmental use of natural clay materials on chromate removal from aqueous solutions. Chem Eng J 168:1024–1031
Erdem E, Karpinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Gode F, Pehlivan E (2003) A comparative study of two chelating ion-exchange resins for the removal of chromium(III) from aqueous solution. J Hazard Mater 100:231–243
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271:321–328
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42:766–770
Gupta SS, Bhattacharyya KG (2006) Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives. J Hazard Mater 128:247–257
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Srivastava SK, Mohan D, Sharma S (1997) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manage 17:517–522
Gupta VK, Mohan D, Sharma S (1998) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mudan aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006b) Adsorption of Safranin-T from wastewater using waste materials— activated carbon and activated rice husks. J Colloid Interface Sci 303:80–86
Gupta VK, Ali I, Saini VK (2007a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Ali I, Saini VK (2007b) Defluoridation of wastewaters using waste carbon slurry. Water Res 41:3307–3316
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Herrero R, Lodeiro P, Rey-Castro C, Vilarin T, Manuel ESV (2005) Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata. Wat Res 39:3199–3210
Hu J, Chen G, Lo IMC (2005) Cr(VI) on megahemite removal and recovery of Cr (VI) from wastewater by megahemite nanoparticles. Wat Res 39:4528–4536
Jiang Y, Pang H, Liao B (2009) Removal of copper(II) ions from aqueous solution by modified bagasse. J Hazard Mater 164:1–9
Lin S, Juang R (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 92:315–326
Mahvi A, Maleki H, Eslami A (2004) A potential of rice husk and rice husk ash for phenol removal in aqueous system. American Appl Sci 1:321–326
Maira AJ, Yeung KL, Soria J, Coronado JM, Belver C, Lee CY, Augugliaro V (2001) Gas-phase photo-oxidation of toluene using nanometer-size TiO2 catalysts. Appl Catal B-Environ 29:327–336
Meenakshi S, Viswanathan N (2007) Identification of selective ion-exchange resin for fluoride sorption. J Colloid Interface Sci 308:438–450
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids Surf A 177:169–181
Nabi D, Aslam I, Qazi IA (2009) Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal. J Environ Sci 21:402–408
Naiya TK, Bhattacharya AK, Das SK (2009) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interface Sci 333:14–26
Ngah WSW, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan–tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manage 91:958–969
Ngah WSW, Kamari A, Koay YJ (2004) Equilibrium and kinetics studies of adsorption of copper(II) on chitosan and chitosan/PVA beads. Int J Biol Macromol 34:155–161
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401–425
Özcan A, Öncü EM, Özcan AS (2006) Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf A 277:90–97
Patterson RR, Fendorf S (1997) Reduction of hexavalent chromium by amorphous iron surface. Environ Sci Technol 31:2039–2044
Rao MV, Patil GR, Borole LV (1998) Effect of mercury and selenite interaction on the mouse vital organs. J Environ Biol 19:215–220
Sampaio RMM, Timmers RA, Xu Y, Keesman KJ, Lens PNL (2009) Selective precipitation of Cu from Zn in a pS controlled continuously stirred tank reactor. J Hazard Mater 165:256–265
Sdiri A, Higashi T, Hatta T, Jamoussi F, Norio T (2010) Mineralogical and spectroscopic characterization, and potential environmental use of limestone from the Abiod formation, Tunisia. Environ Earth Sci 61:1275–1287
Sdiri A, Higashi T, Hatta T, Jamoussi F, Norio T (2011) Evaluating the adsorptive capacity of calcareous and montmorillonitic clays on the removal of several heavy metals in aqueous systems. Chem Eng J 172:37–46
Sdiri A, Higashi T, Chaabouni R, Jamoussi F (2012a) Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays. Water Air Soil Pollut 223:1191–1204
Sdiri A, Higashi T, Jamoussi F, Bouaziz S (2012b) Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J Environ Manage 93:245–253
Shafaei A, Ashtiani FZ, Kaghazchi T (2007) Equilibrium studies of the sorption of Hg(II) ions onto chitosan. Chem Eng J 133:311–316
Shun-Xing L, Feng-Ying Z, Huang Y, Jian-Cong N (2011) Thorough removal of inorganic and organic mercury from aqueous solutions by adsorption on Lemna minor powder. J Hazard Mater 186:423–429
Sreedhar MK, Madhukumar A, Anirudhan TS (1999) Evaluation of an adsorbent prepared by treating coconut husk with polysulphide for the removal of mercury from wastewater. Ind J Eng Mater Sci 6:279–285
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151:1–8
Stephen IB, Wang JS, Lu JF, Siao FY, Chen BH (2009) Adsorption of toxic mercury(II) by an extracellular biopolymer poly(γ-glutamic acid). J Biores Technol 100:200–2007
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Ulmanu M, Marañón E, Fernández Y, Castrillón L, Anger I, Dumitriu D (2003) Removal of copper and cadmium ions from diluted aqueous solutions by low cost and waste material adsorbents. Water Air Soil Pollut 142:357–373
Ünlü N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136:272–280
Yong-Mei H, Man C, Zhong-Bo H (2010) Effective removal of Cu(II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184(392):399
Acknowledgments
We would like to thank Mr. Nidhal Baccar, Technician in the Biotechnology Research Center of Sfax, for his help in the analysis of our samples by atomic absorption spectrometer and his assistance in the laboratory work. The authors would like to extend their thanks to Professor Vinod Kumar Gupta and his team for their prompt reviews and the constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Eloussaief, M., Sdiri, A. & Benzina, M. Modelling the adsorption of mercury onto natural and aluminium pillared clays. Environ Sci Pollut Res 20, 469–479 (2013). https://doi.org/10.1007/s11356-012-0874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0874-4