Abstract
Background, aim, and scope
Textile industry produces wastewater which contributes to water pollution since it utilizes a lot of chemicals. Preliminary studies show that the wastewater from textile industries contains grease, wax, surfactant, and dyes. The objective of this study was to determine the treatment efficiency of the nickel catalysts supported on hydrotalcites in three-dye model compounds and two types of wastewater.
Materials and methods
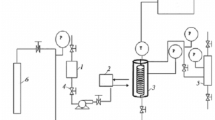
Hydrotalcites were employed to prepare supported nickel catalysts by wetness impregnation technique. Metal loadings from 1 to 10 wt% were tested. Catalysts were characterized by several techniques. They were tested in a catalytic wet air oxidation of three dyes and two wastewaters with different origins.
Results and discussion
It could be observed that the higher the metal content, the lower the BET area, possibly due to sintering of Ni and the consequent blocking of the pores by the metal. In addition, metallic dispersion was also higher when the metal content was lower. Dye conversion was more than 95% for every catalyst showing no differences with the nickel content. A high degree of dye conversion was achieved. Wet air oxidation (WAO) and catalytic wet air oxidation (CWAO) processes have been proved to be extremely efficient in TOC removal for wastewaters.
Conclusions
The CWAO process can be used to remove dyes from wastewater. Three different dyes were tested showing satisfactory results in all of them. TOC degradation and dye removal in the presence of the catalyst were effective. Also, the HTNi catalyst is very active for organic matter and toxicity removal in wastewaters.






Similar content being viewed by others
References
Alejandre A, Medina F, Rodriguez X, Salagre P, Sueiras JE (1999) Preparation and activity of Cu-Al mixed oxides via hydrotalcite-like precursors for the oxidation of phenol aqueous solutions. J Catal 188:311–324
Alzamora L, Ross J, Kruissink E, Van Riejen L (1981) Coprecipitated nickel-alumina catalysts for methanation at high temperature. Part 2. Variation of total and metallic areas as a function of sample composition and method of pretreatment. J Chem Soc Faraday Trans 77:665–681
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53:51–59
Basile F, Benito P, Fornasari G, Vaccari A (2010) Hydrotalcite-type precursors of active catalysts for hydrogen production. Appl Clay Sci 48:250–259
Do D (1998) Adsorption analysis: equilibria and kinetics. Imperial College Press, London
Eaton AD, Clesceri LS, Rice EW, Greenberg AE, Franson MAH (eds) (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC
Esplugas S, Giménez J, Contreras S, Pascual S, Rodríguez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36:1034–1042
García J, Gomes HT, Serp P, Kalck P, Figuereido JL, Faria JL (2005) Platinum catalysts supported on MWNT for catalytic wet air oxidation of nitrogen containing compounds. Catal Today 102:101–109
Kawabata T, Shinozuka Y, Ohishi Y, Shishido T, Takaki K, Takehira K (2005) Nickel containing Mg-Al hydrotalcite-type anionic clay catalyst for the oxidation of alcohols with molecular oxygen. J Mol Catal A Chem 236:206–215
Kirm I, Medina F, Rodríguez X, Cesteros Y, Salagre P, Sueiras J (2004) Epoxidation of styrene with hydrogen peroxide using hydrotalcites as heterogeneous catalysts. Appl Catal A Gen 272:175–185
Levec J, Pintar A (2007) Catalytic wet-air oxidation processes: a review. Catal Today 124:172–184
Mishra VS, Mahajani VV, Joshi JB (1995) Wet air oxidation. Ind Eng Chem Res 34:2–48
Ohishi Y, Kawabata T, Shishido T, Takaki K, Zhang Q, Wang Y, Nomura K, Takehira K (2005) Mg-Fe-Al mixed oxides with mesoporous properties prepared from hydrotalcite as precursors: catalytic behaviour in ethylbenzene dehydrogenation. Appl Catal A Gen 288:220–234
Ovejero G, Sotelo JL, Romero MD, Rodríguez A, Ocaña MA, Rodríguez G, García J (2006) Multiwalled carbon nanotubes for liquid-phase oxidation functionalization, characterization and catalytic activity. Ind Eng Chem Res 45:2206–2212
Ovejero G, Rodríguez A, Vallet A, García J (2011) Studies in catalytic wet air oxidation as a process to destroy CI Basic Yellow 11 in aqueous stream over platinum catalyst. Color Technol 127:10–17
Palacio L, Velasquez J, Echavarría A, Faro A, Ribeiro F, Ribeiro M (2010) Total oxidation of toluene over calcined trimetallic hydrotalcites type catalysts. J Hazard Mater 177:407–413
Papic S, Koprivanac N, Loncaric B, Metes A (2004) Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigm 62:291–298
Pintar A, Besson M, Gallezot P, Durecu S (2001) Catalytic wet air oxidation of Kraft bleach plant effluents in a trickle-bed reactor over a Ru/TiO2 catalyst. Appl Catal B Environ 31:275–290
Rajkumar D, Song BJ, Kim JG (2007) Electrochemical degradation of Reactive Blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds. Dyes Pigm 72:1–7
Rodríguez A, Ovejero G, Romero MD, Díaz C, Barreiro M, García J (2008) Catalytic wet air oxidation of textile industrial wastewater using metal supported on carbon nanofibers. J Supercrit Fluids 46:163–172
Schulze K, Makowski W, Chyzy R, Dziembaj R, Geismar G (2001) Nickel doped hydrotalcites as catalyst precursors for the partial oxidation of light paraffins. Appl Clay Sci 18:59–69
Shiraga M, Kawabata T, Li D, Shishido T, Komaguchi K, Sano T, Takehira K (2006) Memory effect-enhanced catalytic ozonation of aqueous phenol and oxalic acid over supported Cu catalysts derived from hydrotalcite. Appl Clay Sci 33:247–259
Tichit D, Medina F, Coq B, Dutartre R (1997) Activation under oxidizing and reducing atmospheres of Ni-containing layered double oxides. Appl Catal A Gen 159:241–258
Acknowledgments
The authors gratefully acknowledge the financial support from Ministerio de Educación y Ciencia by CONSOLIDER Program through TRAGUA Network CSD2006-44, CTQ2008-02728, and by Comunidad de Madrid through REMTAVARES Network S-2009/AMB-1588.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Ovejero, G., Sotelo, J.L., Rodríguez, A. et al. Wet air oxidation and catalytic wet air oxidation for dyes degradation. Environ Sci Pollut Res 18, 1518–1526 (2011). https://doi.org/10.1007/s11356-011-0504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0504-6