Abstract
Purpose
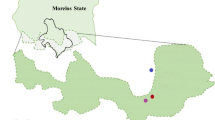
We tested the genetic diversity in wild mice (Mus musculus domesticus) inhabiting the asbestos-polluted area as a model for the long-term mutagenic effect of asbestos. Hazardous effects of deposited asbestos persist in the environment because of low rate of fiber disintegration. The upper layers of the soil in the vicinity of a former asbestos factory are nearly “saturated” with asbestos fibers and dust. Natural populations of mice dwell in this area and are constantly exposed to asbestos fibers.
Methods
We measured the microsatellites genetic diversity of wild mice (Mus musculus domesticus) inhabiting the asbestos-polluted area as a model for the long-term mutagenic effect of this environmental toxin.
Results
The six tested microsatellites were highly polymorphic, revealing 111 different alleles for the two sampled populations. Effective number of alleles was slightly higher in the polluted population relative to the control population, while observed heterozygosity was lower. The chromatographic profile of the polluted population exhibited a significantly higher number of bands, probably resulting from somatic mutations, in addition to the ordinary microsatellite band profiles.
Conclusions
Long-term exposure to asbestos fibers significantly elevates the level of somatic mutations. It also leads to a relatively high level of observed homozygosity, a phenomenon that may be associated with loss of heterozygosity. Based on the mice population, our data suggest elevated health risks for humans living in an asbestos-polluted area.

Similar content being viewed by others
References
Ben-Shlomo R, Nevo E (1988) Isozyme polymorphism as monitoring of marine environments: the interactive effect of cadmium and mercury pollution on the shrimp, Palaemon elegans. Mar Pollut Bull 19:314–317
Ben-Shlomo R, Neufeld E, Berger D, Lenington S, Ritte U (2007) The dynamic of t-haplotypes in wild populations of the house mouse (Mus domesticus) in Israel. Mamm Genome 18:164–172
Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R (2000) Effects of chemical contaminants on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res 463:33–51
Both K, Henderson DW, Turner DR (1994) Asbestos and erionite fibres can induce mutations in human lymphocytes that result in loss of heterozygosity. Int J Cancer 59:538–542
Both K, Turner DR, Henderson DW (1995) Loss of heterozygosity in asbestos-induced mutations in a human mesothelioma cell line. Environ Mol Mutagen 26:67–71
Carter RS, Avadhani NG (1991) Cloning and characterization of the mouse cytochrome c oxidase subunit IV gene. Arch Biochem Biophys 288:97–106
Daniel FB (1983) In vitro assessment of asbestos genotoxicity. Environ Health Persp 53:163–167
Dizenhouse D, Verga Y (2002) Asbestos survey in the Western Galilee, submitted to the Israeli Ministry of Environment. In Hebrew. pp 1–283
Dubrova YE (2005) Radiation-induced mutation at tandem repeat DNA loci in the mouse germline: spectra and doubling doses. Radiat Res 163:200–207
Dubrova YE, Plumb MA (2002) Ionising radiation and mutation induction at mouse minisatellite loci the story of the two generations. Mutat Res 499:143–150
Dubrova Y, Neumann R, Leidl DL, Jeffreys A, Nesterov VN, Krouchinsky NG, Ostapenko VA (1996) Human minisatellite mutation rate after the chernobyl accident. Nature 380:683–686
Ellegren H, Lindgren G, Primmer CR, Moller AP (1997) Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature 389:593–596
Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G et al (2007) Patterns of somatic mutation in human cancer genomes. Nature 446:153–158
Guttman SI (1994) Population genetic structure and ecotoxicology. Environ Health Persp 102:97–100
Jaurand MC (1997) Mechanisms of fiber-induced genotoxicity. Environ Health Persp 105(Suppl 5):1073–1084
Kettunen E, Aavikko M, Nymark P, Ruosaari S, Wikman H, Vanhala E et al (2009) DNA copy number loss and allelic imbalance at 2p16 in lung cancer associated with asbestos exposure. Brit J Cancer 100:1336–1342
LaDou J (2004) The asbestos cancer epidemic. Environ Health Persp 112:285–290
Lawson PR, Perkins VC, Holmskov U, Reid KB (1999) Genomic organization of the mouse gene for lung surfactant protein D. Am J Resp Cell Mol 20:953–963
Love JM, Knight AM, McAleer MA, Todd JA (1990) Toward construction of a high resolution map of the mouse genome using PCR-analyzed microsatellites. Nuc Acids Res 18:4123–4130
Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics 142:1061–1064
Ministry of the Environment—State of Israel (www.environment.gov.il) Confronting the asbestos problem in Israel. 2005:1–8
Nakamura K, Xiu Y, Ohtsuji M, Sugita G, Abe M, Ohtsuji N et al (2003) Genetic dissection of anxiety in autoimmune disease. Hum Mol Genet 12:1079–1086
Nevo E, Ben-Shlomo R, Lavie B (1984) Mercury selection of allozymes in marine organisms: prediction and verification in nature. Proc Natl Acad Sci USA 81:1258–1259
Nymark P, Kettunen E, Aavikko M, Ruosaari S, Kuosma E, Vanhala E et al (2009) Molecular alterations at 9q33.1 and polyploidy in asbestos-related lung cancer. Clin Cancer Res 15:468–475
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in excel population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Ruosaari ST, Nymark PEH, Aavikko MM, Kettunen E, Knuutila S, Hollmen J et al (2008) Aberrations of chromosome 19 in asbestos-associated lung cancer and in asbestos-induced micronuclei of bronchial epithelial cells in vitro. Carcinogenesis 29:913–917
Somers CM (2006) Expanded simple tandem repeat (ESTR) mutation induction in the male germline: lessons learned from lab mice. Mutat Res 598:35–49
Theodorakis CW (2001) Integration of genotoxic and population genetic endpoints in biomonitoring and risk assessment. Ecotoxicology 10:245–256
Topinka J, Loli P, Georgiadis P, Dušinská M, Hurbánková M, Kováciková Z, Volkovová K, Kažimırová A, Barancoková M, Tatrai E, Oesterle D, Wolff T, Kyrtopoulos SA (2004) Mutagenesis by asbestos in the lung of λ-lacI transgenic rats. Mutat Res 553:67–78
Xu A, Zhou H, Yu DZ, Hei TK (2002) Mechanisms of the genotoxicity of crocidolite asbestos in mammalian cells: Implication from mutation patterns induced by reactive oxygen species. Environ Health Persp 110:1003–1008
Xu A, Huang X, Lien YC, Bao L, Yu Z, Hei TK (2007a) Genotoxic mechanisms of asbestos fibers: role of extranuclear targets. Chem Res Toxicol 20:724–733
Xu A, Smilenov LB, He P, Masumura K, Nohmi T, Yu Z, Heil TK (2007b) New insight into intrachromosomal deletions induced by chrysotile in the gpt delta transgenic mutation assay. Environ Health Persp 115:87–92
Yauk CL, Quinn JS (1996) Multilocus DNA fingerprinting reveals high rate of heritable genetic mutation in herring gulls nesting in an industrialized urban site. Proc Natl Acad Sci USA 93:12137–12141
Yauk CL, Fox GA, McCarry BE, Quinn JS (2000) Induced minisatellite germline mutations in herring gulls (Larus argentatus) living near steel mills. Mutat Res 452:211–218
Yauk CL, Dubrova YE, Grant GR, Jeffreys AJ (2002) A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutat Res 500:147–156
Acknowledgments
We thank K. Lidenfeld and T. Shaked for their assistance. We would like to express our thanks to the anonymous referees, whose helpful suggestions contributed significantly to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Ben-Shlomo, R., Shanas, U. Genetic ecotoxicology of asbestos pollution in the house mouse Mus musculus domesticus . Environ Sci Pollut Res 18, 1264–1269 (2011). https://doi.org/10.1007/s11356-011-0481-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0481-9