Abstract
Background, aim, and scope
Nanomaterials have been used increasingly in industrial production and daily life, but their human exposure may cause health risks. The interactions of nanomaterial with functional biomolecules are often applied as a precondition for its cytotoxicity and organ toxicity where various proteins have been investigated in the past years. In the present study, nano-TiO2 was selected as the representative of nanomaterials and lysozyme as a representative for enzymes. By investigating their interaction by various instrumentations, the objective is to identify the action sites and types, estimate the effect on the enzyme structure and activity, and reveal the toxicity mechanism of nanomaterial.
Materials and methods
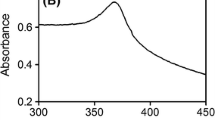
Laboratory-scale experiments were carried out to investigate the interactions of nano-TiO2 with lysozyme. The interaction of nano-TiO2 particles with lysozyme has been studied in the analogous physiological media in detail by UV spectrometry, fluorophotometry, circular dichroism (CD), scanning electron microscope, ζ-potential, and laser particle size.
Results
The interaction accorded with the Langmuir isothermal adsorption and the saturation number of lysozyme is determined to be 580 per nano-TiO2 particle (60 nm of size) with 4.7 × 106 M−1 of the stability constant in the physiological media. The acidity and ion strength of the media obviously affected the binding of lysozyme. The warping and deformation of the lysozyme bridging were demonstrated by the conversion of its spatial structure from α-helix into a β-sheet, measured by CD. In the presence of nano-TiO2, the bacteriolysis activity of lysozyme was subjected to an obvious inhibition.
Discussion
The two-step binding model of lysozyme was proposed, in which lysozyme was adsorbed on nano-TiO2 particle surface by electrostatic interaction and then the hydrogen bond (N–H···O and O–H···O) formed between nano-TiO2 particle and polar side groups of lysozyme. The adsorption of lysozyme obeyed the Langmuir isothermal model. The binding of lysozyme is dependent on the acidity and ion strength of the media. The bigger TiO2 aggregate was formed in the presence of lysozyme where lysozyme may bridge between nano-TiO2 particles. The coexistence of nano-TiO2 particles resulted in the transition of lysozyme conformation from an α-helix into a β-sheet and a substantial inactivation of lysozyme. The β-sheet can induce the formation of amyloid fibrils, a process which plays a major role in pathology.
Conclusions
Lysozyme was adsorbed on the nano-TiO2 particle surface via electrostatic attraction and hydrogen bonds, and they also bridged among global nano-TiO2 particles to form the colloidal particles. As a reasonable deduction of this study, nano-TiO2 might have some toxic impacts on biomolecules. Our data suggest that careful attention be paid to the interaction of protein and nanomaterials. This could contribute to nanomaterial toxicity assessment.
Recommendations and perspectives
Our results strongly suggest that nano-TiO2 has an obvious impact on biomolecules. Our data suggest that more attention should be paid to the potential toxicity of nano-TiO2 on biomolecules. Further research into the toxicity of nanosized particles needs to be carried out prior to their cell toxicity and tissue toxicity. These investigations might serve as the basis for determining the toxicity and application of nanomaterials.








Similar content being viewed by others
References
Buzea C, Pacheco I, Robbie K (2006) Nanomaterials—sources, classification, and toxicity. Comp Biochem Physiol A 143(4):S123–S123
Chen FF, Tang YN, Wang SL, Gao HW (2008) Binding of brilliant red compound to lysozyme: insights into the enzyme toxicity of water-soluble aromatic chemicals. Amino Acids 36(3):399–407
Chen XH, Zhang L, Weng YX, Du LC, Ye MP, Yang GZ, Fujii R, Rondonuwu FS, Koyama Y, Wu YS, Zhang JP (2005) Protein structural deformation induced lifetime shortening of photosynthetic bacteria light-harvesting complex LH2 excited state. Biophys J 88(6):4262–4273
Chen YL, Zhang XF, Gong YD, Zhao NM, Zeng TY, Song XQ (1999) Conformational changes of fibrinogen adsorption onto hydroxyapatite and titanium oxide nanoparticles. J Colloid Interface Sci 214(1):38–45
Derham BK, Harding JJ (2006) The effect of the presence of globular proteins and elongated polymers on enzyme activity. Biochim Biophys Acta 1764(6):1000–1006
Donaldson K, Stone V, Seaton A, MacNee W (2001) Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 109:523–527
Dunford R, Salinaro A, Cai LZ, Serpone N, Horikoshi S, Hidaka H, Knowland J (1997) Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett 418(1–2):87–90
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455(1–2):81–95
Gao HW (1999) Establishment of SS absorption in suspension liquid. Asian J Chem 11(4):1556–1558
Gao HW, Zhao JF, Yang QZ, Liu XH, Chen L, Pan LT (2006) Non-covalent interaction of 2′,4′,5′,7′-tetrabromo-4,5,6,7-tetrachlorofluorescein with proteins and its application. Proteomics 6(19):5140–5151
Gao HW, Xu Q, Chen L, Wang SL, Wang Y, Wu LL, Yuan Y (2008) Potential protein toxicity of synthetic pigments: binding of poncean S to human serum albumin. Biophys J 94(3):906–917
Giacomelli CE, Avena MJ, DePauli CP (1997) Adsorption of bovine serum albumin onto TiO2 particles. J Colloid Interface Sci 188(2):387–395
Goto Y, Yagi H, Yamaguchi K, Chatani E, Ban T (2008) Structure, formation and propagation of amyloid fibrils. Curr Pharm Des 14(30):3205–3218
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71(7):1308–1316
Hu HY, Xu GJ (1999) Structural transformation of proteins. Prog Biochem Biophys 26(1):9–12
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ Sci Pollut Res 13(4):225–232
Lee YC, Yang D (2002) Determination of lysozyme activities in a microplate format. Anal Biochem 310(2):223–224
Li L, Gao HW, Ren JR, Chen L, Li YC, Zhao JF, Zhao HP, Yuan Y (2007) Binding of Sudan II and IV to lecithin liposomes and E. coli membranes: insights into the toxicity of hydrophobic azo dyes. BMC Struct Biol 7:6
Liu HS, Wang YC, Chen WY (1995) The sorption of lysozyme and ribonuclease onto ferromagnetic nickel powder 1. Adsorption of single components. Colloids Surf B 5(1–2):25–34
Liu ST, Sugimoto T, Azakami H, Kato A (2000) Lipophilization of lysozyme by short and middle chain fatty acids. J Agric Food Chem 48(2):265–269
Lovern SB, Strickler JR, Klaper R (2007) Behavioral and physiological changes in daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol 41(12):4465–4470
Lu NH, Zhu ZN, Zhao XQ, Tao R, Yang XL, Gao ZH (2008) Nano titanium dioxide photocatalytic protein tyrosine nitration: a potential hazard of TiO2 on skin. Biochem Biophys Res Commun 370(4):675–680
Lundqvist M, Sethson I, Jonsson BH (2004) Protein adsorption onto silica nanoparticles: conformational changes depend on the particles' curvature and the protein stability. Langmuir 20(24):10639–10647
Merlini G, Bellotti V (2005) Lysozyme: a paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin Chim Acta 357(2):168–172
Oliva FY, Avalle LB, Camara OR, De Pauli CP (2003) Adsorption of human serum albumin (HSA) onto colloidal TiO2 particles, part I. J Colloid Interface Sci 261(2):299–311
Roddick-Lanzilotta AD, Connor PA, McQuillan AJ (1998) An in situ infrared spectroscopic study of the adsorption of lysine to TiO2 from an aqueous solution. Langmuir 14(22):6479–6484
Sanderson BJS, Wang JJ, Wang H (2007) Letter and new data in response to Letter to the Editor by William P. Gulledge about article “Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells” by Wang et al. Reply. Mutat Res 634(1–2):243–244
Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D (2007) Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res 18(5):630–638
Simon A, Gouget B, Mayne M, Herlin N, Reynaud C, Degrouard J, Carriere M (2007) In vitro investigation of TiO2, Al2O3, Au nanoparticles and multi-walled carbon nanotubes cyto- and genotoxicity on lung, kidney cells and hepatocytes. Toxicol Lett 172:S36–S36
Stone V, Johnston H, Clift MJD (2007) Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Transactions on NanoBioscience 6(4):331–340
Turkan Kopac KB, Yener J (2008) Effect of pH and temperature on the adsorption of bovine serum albumin onto titanium dioxide. Colloids Surf A Physicochem Eng Asp 322(2008):19–28
Van Dael H (1998) Chimeras of human lysozyme and alpha-lactalbumin: an interesting tool for studying partially folded states during protein folding. Cell Mol Life Sci 54(11):1217–1230
Voros J (2004) The density and refractive index of adsorbing protein layers. Biophys J 87(1):553–561
Wang JJ, Sanderson BJS, Wang H (2007) Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res 628(2):99–106
Zhu MT, Feng WY, Wang B, Wang TC, Gu YQ, Wang M, Wang Y, Ouyang H, Zhao YL, Chai ZF (2008) Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology 247(2–3):102–111
Acknowledgements
This work was supported by the National Major Project of Science and Technology Ministry of China (no. 2008ZX07421-002) and the State Key Laboratory Foundation of Science and Technology Ministry of China (grant no. PCRRK08003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Stefan Trapp
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 60.3 kb)
Rights and permissions
About this article
Cite this article
Xu, Z., Liu, XW., Ma, YS. et al. Interaction of nano-TiO2 with lysozyme: insights into the enzyme toxicity of nanosized particles. Environ Sci Pollut Res 17, 798–806 (2010). https://doi.org/10.1007/s11356-009-0153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0153-1