Abstract
Background
Rupture of brain aneurysms is associated with high fatality and morbidity rates. Through remodeling of the collagen matrix, many aneurysms can remain unruptured for decades, despite an enlarging and evolving geometry.
Objective
Our objective was to explore this adaptive remodeling for the first time in an elastase induced aneurysm model in rabbits.
Methods
Saccular aneurysms were created in 22 New Zealand white rabbits and remodeling was assessed in tissue harvested 2, 4, 8 and 12 weeks after creation.
Results
The intramural principal stress ratio doubled after aneurysm creation due to increased longitudinal loads, triggering a remodeling response. A distinct wall layer with multi-directional collagen fibers developed between the media and adventitia as early as 2 weeks, and in all cases by 4 weeks with an average thickness of 50.6 ± 14.3 μm. Collagen fibers in this layer were multi-directional (AI = 0.56 ± 0.15) with low tortuosity (1.08 ± 0.02) compared with adjacent circumferentially aligned medial fibers (AI = 0.78 ± 0.12) and highly tortuous adventitial fibers (1.22 ± 0.03). A second phase of remodeling replaced circumferentially aligned fibers in the inner media with longitudinal fibers. A structurally motivated constitutive model with both remodeling modes was introduced along with methodology for determining material parameters from mechanical testing and multiphoton imaging.
Conclusions
A new mechanism was identified by which aneurysm walls can rapidly adapt to changes in load, ensuring the structural integrity of the aneurysm until a slower process of medial reorganization occurs. The rabbit model can be used to evaluate therapies to increase aneurysm wall stability.












Similar content being viewed by others
References
Raghavan ML, Ma B, Harbaugh RE (2005) Quantified aneurysm shape and rupture risk. J Neurosurg 102:355–362. https://doi.org/10.3171/jns.2005.102.2.0355
Juvela S, Porras M, Poussa K (2000) Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 93:379–387. https://doi.org/10.3171/jns.2000.93.3.0379
Fennell VS, Kalani MYS, Atwal G, Martirosyan NL, Spetzler RF (2016) Biology of Saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg 3:43. https://doi.org/10.3389/fsurg.2016.00043
Naggara ON, Lecler A, Oppenheim C, Meder JF, Raymond J (2012) Endovascular treatment of intracranial unruptured aneurysms: a systematic review of the literature on safety with emphasis on subgroup analyses. Radiology 263:828–835. https://doi.org/10.1148/radiol.12112114
Kotowski M, Naggara O, Darsaut TE, Nolet S, Gevry G, Kouznetsov E, Raymond J (2013) Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry 84:42–48. https://doi.org/10.1136/jnnp-2011-302068
Nahed BV, DiLuna ML, Morgan T, Ocal E, Hawkins AA, Ozduman K, Kahle KT, Chamberlain A, Amar AP, Gunel M (2005) Hypertension, age, and location predict rupture of small intracranial aneurysms. Neurosurgery 57:676–683. https://doi.org/10.1093/neurosurgery/57.4.676
Detmer FJ, Chung BJ, Jimenez C, Hamzei-Sichani F, Kallmes D, Putman C, Cebral JR (2019) Associations of hemodynamics, morphology, and patient characteristics with aneurysm rupture stratified by aneurysm location. Neuroradiology 61:275–284. https://doi.org/10.1007/s00234-018-2135-9
Detmer FJ, Mut F, Slawski M, Hirsch S, Bijlenga P, Cebral JR (2020) Incorporating variability of patient inflow conditions into statistical models for aneurysm rupture assessment. Acta Neurochir 162:553–566. https://doi.org/10.1007/s00701-020-04234-8
Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W (2018) Vessel Wall Imaging of Intracranial Aneurysms: Systematic Review and Meta-analysis. World Neurosurg. 117:453–458. https://doi.org/10.1016/j.wneu.2018.06.008
Wang G, Li W, Lei S, Ge X, dong, Yin J bo, Zhang D, (2019) Relationships between aneurysmal wall enhancement and conventional risk factors in patients with intracranial aneurysm: A high-resolution MRI study. J Neuroradiol 46:25–28. https://doi.org/10.1016/j.neurad.2018.09.007
Quan K, Song J, Yang Z, Wang D, An Q, Huang L, Liu P, Li P, Tian Y, Zhou L, Zhu W (2019) Validation of wall enhancement as a new imaging biomarker of Unruptured cerebral aneurysm. Stroke 50:1570–1573. https://doi.org/10.1161/STROKEAHA.118.024195
Vakil P, Ansari SA, Cantrell CG, Eddleman CS, Dehkordi FH, Vranic J, Hurley MC, Batjer HH, Bendok BR, Carroll TJ (2015) Quantifying intracranial Aneurysm Wall permeability for risk assessment using dynamic contrast-enhanced MRI: a pilot study. AJNR Am J Neuroradiol 36:953–959. https://doi.org/10.3174/ajnr.A4225
King RM, Marosfoi M, Caroff J, Ughi GJ, Groth DM, Gounis MJ, Puri AS (2019) High frequency optical coherence tomography assessment of homogenous neck coverage by intrasaccular devices predicts successful aneurysm occlusion. J Neurointerv Surg 11:1150–1154. https://doi.org/10.1136/neurintsurg-2019-014843
Hartmann K, Stein KP, Neyazi B, Erol Sandalcioglu I (2019) Aneurysm architecture: first in vivo imaging of human cerebral aneurysms with extravascular optical coherence tomography. Cerebrovasc Dis 48:26–31. https://doi.org/10.1159/000502450
Lall RR, Eddleman CS, Bendok BR, Batjer HH (2009) Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus 26:E2. https://doi.org/10.3171/2009.2.focus0921
Robertson AM, Duan X, Aziz KM, Hill MR, Watkins SC, Cebral JR (2015) Diversity in the strength and structure of unruptured cerebral aneurysms. Ann Biomed Eng 43:1502–1515. https://doi.org/10.1007/s10439-015-1252-4
Cebral JR, Duan X, Chung BJ, Putman C, Aziz K, Robertson AM (2015) Wall mechanical properties and hemodynamics of Unruptured intracranial aneurysms. Am J Neuroradiol 36:1695–1703. https://doi.org/10.3174/ajnr.a4358
Cebral JR, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR, Tulamo R, Hernesniemi J, Niemelä M, Robertson A, Frösen J (2017) Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. Am J Neuroradiol 38:119–126. https://doi.org/10.3174/ajnr.A4951
Robertson AM, Watton PN (2013) Mechanobiology of the Arterial Wall. In: Becker S, Kuznetsov A (eds) Transport in biological media. Elsevier, New York, pp 275–347
Stehbens WE (1963) Histopathology of cerebral aneurysms. Arch Neurol 8:272–285. https://doi.org/10.1001/archneur.1963.00460030056005
Yong-Zhong G, Van Alphen HAM (1990) Pathogenesis and histopathology of saccular aneurysms: review of the literatutre. Neurol Res 12:249–255. https://doi.org/10.1080/01616412.1990.11739952
Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, Gonzalez NR, Sayre J, Vinuela FV (2013) Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology 269:258–265. https://doi.org/10.1148/radiol.13121188
Schneiders JJ, Marquering HA, Van Den Berg R, VanBavel E, Velthuis B, Rinkel GJE, Majoie CB (2014) Rupture-associated changes of cerebral aneurysm geometry: High-resolution 3D imaging before and after rupture. Am J Neuroradiol 35:1358–1362. https://doi.org/10.3174/ajnr.A3866
Vlak MHM, Rinkel GJE, Greebe P, van der Bom JG, Algra A (2011) Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke 42:1878–1882. https://doi.org/10.1161/STROKEAHA.110.606558
Björkman J, Frösen J, Tähtinen O, Backes D, Huttunen T, Harju J, Huttunen J, Kurki MI, von und zu Fraunberg M, Koivisto T, Manninen H, Jääskeläinen JE, Lindgren AE (2017) Irregular shape identifies ruptured intracranial aneurysm in subarachnoid hemorrhage patients with multiple aneurysms. Stroke 48:1986–1989. https://doi.org/10.1161/STROKEAHA.117.017147
Humphrey JD, Rajagopal K (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Model Methods Appl Sci 12:407–430. https://doi.org/10.1142/S0218202502001714
Baek S, Rajagopal KR, Humphrey JD (2006) A theoretical model of enlarging intracranial fusiform aneurysms. J Biomech Eng 128:142–149. https://doi.org/10.1115/1.2132374
Watton PN, Ventikos Y, Holzapfel GA (2009) Modelling the growth and stabilization of cerebral aneurysms. Math Med Biol 26:133–164. https://doi.org/10.1093/imammb/dqp001
Gade P (2019) Coupled theoretical and experimental methods to understand growth and remodeling of in situ engineered vascular grafts in young and aged hosts. Doctoral Dissertation, University of Pittsburgh
Khosravi R, Miller KS, Best CA, Shih YC, Lee Y-U, Yi T, Shinoka T, Breuer CK, Humphrey JD (2015) Biomechanical Diversity Despite Mechanobiological Stability in Tissue Engineered Vascular Grafts Two Years Post-Implantation. Tissue Eng Part A 21:1529–1538. https://doi.org/10.1089/ten.tea.2014.0524
Doillon CJ, Dunn MG, Bender E, Silver FH (1985) Collagen Fiber Formation in Repair Tissue: Development of Strength and Toughness. Coll Relat Res 5:481–492. https://doi.org/10.1016/S0174-173X(85)80002-9
Zeng Z, Kallmes DF, Durka MJ, Ding Y, Lewis D, Kadirvel R, Robertson AM (2011) Hemodynamics and anatomy of elastase-induced rabbit aneurysm models: similarity to human cerebral aneurysms? Am J Neuroradiol 32:595–601. https://doi.org/10.3174/ajnr.A2324
Wang S, Dai D, Kolumam Parameswaran P, Kadirvel R, Ding Y-H, Robertson AM, Kallmes DF (2018) Rabbit aneurysm models mimic histologic wall types identified in human intracranial aneurysms. J Neurointerv Surg 10:411–415. https://doi.org/10.1136/neurintsurg-2017-013264
Altes TA, Cloft HJ, Short JG, DeGast A, Do HM, Helm GA, Kallmes DF (2000) Creation of Saccular aneurysms in the rabbit. Am J Roentgenol 174:349–354. https://doi.org/10.2214/ajr.174.2.1740349
Ghasemi M, Nolan DR, Lally C (2018) An investigation into the role of different constituents in damage accumulation in arterial tissue and constitutive model development. Biomech Model Mechanobiol 17:1757–1769. https://doi.org/10.1007/s10237-018-1054-3
Stemper BD, Yoganandan N, Stineman MR, Gennarelli TA, Baisden JL, Pintar FA (2007) Mechanics of fresh, refrigerated, and frozen arterial tissue. J Surg Res 139:236–242. https://doi.org/10.1016/j.jss.2006.09.001
Cheng F, Birder LA, Kullmann FA, Hornsby J, Watton PN, Watkins S, Thompson M, Robertson AM (2018) Layer-dependent role of collagen recruitment during loading of the rat bladder wall. Biomech Model Mechanobiol 17:403–417. https://doi.org/10.1007/s10237-017-0968-5
Humphrey JD, Canham PB (2000) Structure, mechanical properties, and mechanics of intracranial saccular aneurysms. J Elast 61:49–81. https://doi.org/10.1023/A:1010989418250
Sang C, Maiti S, Fortunato R, Kofler J, Robertson AM (2018) a Uniaxial Testing Approach for Consistent Failure in Vascular Tissues. J Biomech Eng 140:061010. https://doi.org/10.1115/1.4039577
Hill MR, Duan X, Gibson GA, Watkins S, Robertson AM (2012) A theoretical and non-destructive experimental approach for direct inclusion of measured collagen orientation and recruitment into mechanical models of the artery wall. J Biomech 45:762–771. https://doi.org/10.1016/j.jbiomech.2011.11.016
Bredfeldt JS, Liu Y, Pehlke CA, Conklin MW, Szulczewski JM, Inman DR, Keely PJ, Nowak RD, Mackie TR, Eliceiri KW (2014) Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J Biomed Opt 19:016007. https://doi.org/10.1117/1.JBO.19.1.016007
Liu Y, Keikhosravi A, Mehta GS, Drifka CR, Eliceiri KW (2017) Methods for Quantifying Fibrillar Collagen Alignment. In: Rittié L (ed) Fibrosis. Humana Press, New York, NY, pp 429–451. https://doi.org/10.1007/978-1-4939-7113-8_28
Sander EA, Barocas VH (2009) Comparison of 2D fiber network orientation measurement methods. J Biomed Mater Res - Part A 88:322–331. https://doi.org/10.1002/jbm.a.31847
Parshin DV, Lipovka AI, Yunoshev AS, Ovsyannikov KS, Dubovoy AV, Chupakhin AP (2019) On the optimal choice of a hyperelastic model of ruptured and unruptured cerebral aneurysm. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-52229-y
Raghavan ML, Kratzberg J, Castro de Tolosa EM, Hanaoka MM, Walker P, da Silva ES (2006) Regional distribution of wall thickness and failure properties of human abdominal aortic aneurysm. J Biomech 39:3010–3016. https://doi.org/10.1016/j.jbiomech.2005.10.021
Lu J, Hu S, Raghavan ML (2013) A shell-based inverse approach of stress analysis in intracranial aneurysms. Ann Biomed Eng 41:1505–1515. https://doi.org/10.1007/s10439-013-0751-4
Betsch P, Gruttmann F, Stein E (1996) A 4-node finite shell element for the implementation of general hyperelastic 3D-elasticity at finite strains. Comput Methods Appl Mech Eng 130:57–79. https://doi.org/10.1016/0045-7825(95)00920-5
Maas SA, Ellis BJ, Ateshian GA, Weiss JA (2012) FEBio: finite elements for biomechanics. J Biomech Eng 134:011005. https://doi.org/10.1115/1.4005694
Kurashina T, Sakamaki T, Yagi A, Nakamura Tetsuya Sakamoto, Hironosuke Nushiro N (1994) A new device for indirect blood pressure measurement in rabbits. Jpn Circ J 58:264–268. https://doi.org/10.1253/jcj.58.264
Lanir Y (1994) Plausibility of structral constitutive equations for isotropic soft tissues in finite static deformations. J Appl Mech 61:695–702. https://doi.org/10.1115/1.2901516
Schriefl AJ, Reinisch AJ, Sankaran S, Pierce DM, Holzapfel GA (2012) Quantitative assessment of collagen fibre orientations from two-dimensional images of soft biological tissues. J R Soc Interface 9:3081–3093. https://doi.org/10.1098/rsif.2012.0339
Wulandana R, Robertson AM (2005) An inelastic multi-mechanism constitutive equation for cerebral arterial tissue. Biomech Model Mechanobiol 4:235–248. https://doi.org/10.1007/s10237-005-0004-z
Li D, Robertson AM (2009) A structural multi-mechanism constitutive equation for cerebral arterial tissue. Int J Solids Struct 46:2920–2928. https://doi.org/10.1016/j.ijsolstr.2009.03.017
Cebral JR, Raschi M (2013) Suggested connections between risk factors of intracranial aneurysms: a review. Ann Biomed Eng 41:1366–1383. https://doi.org/10.1007/s10439-012-0723-0
Watton PN, Selimovic A, Raberger NB, Huang P, Holzapfel GA, Ventikos Y (2011) Modelling evolution and the evolving mechanical environment of saccular cerebral aneurysms. Biomech Model Mechanobiol 10:109–132. https://doi.org/10.1007/s10237-010-0221-y
Zeinali-Davarani S, Chow M-J, Turcotte R, Zhang Y (2013) Characterization of biaxial mechanical behavior of porcine aorta under gradual elastin degradation. Ann Biomed Eng 41:1528–1538. https://doi.org/10.1007/s10439-012-0733-y
Langille BL, Bendeck MP, Keeley FW (1989) Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol Circ Physiol 256:H931–H939. https://doi.org/10.1152/ajpheart.1989.256.4.h931
Hayashi K, Makino A, Kakoi D (2018) Remodeling of arterial wall : response to changes in both blood flow and blood pressure. J Mech Behav Biomed Mater 77:475–484
Jackson ZS, Gotlieb AI, Langille BL (2002) Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res 90:918–925. https://doi.org/10.1161/01.RES.0000016481.87703.CC
Gleason RL, Wilson E, Humphrey JD (2007) Biaxial biomechanical adaptations of mouse carotid arteries cultured at altered axial extension. J Biomech 40:766–776. https://doi.org/10.1016/j.jbiomech.2006.03.018
Duan X (2016) The link between hemodynamics and wall structure in cerebral aneurysms. Doctoral Dissertation, University of Pittsburgh
Cicchi R, Pavone FS, Massi D, Sampson DD (2005) Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents. Opt Express 13:2337–2344. https://doi.org/10.1364/opex.13.002337
Genina EA, Bashkatov AN, Tuchin VV (2010) Tissue optical immersion clearing. Expert Rev Med Devices 7:825–842. https://doi.org/10.1586/erd.10.50
Marosfoi M, Langan ET, Strittmatter L, van der Marel K, Vedantham S, Arends J, Lylyk IR, Loganathan S, Hendricks GM, Szikora I, Puri AS, Wakhloo AK, Gounis MJ (2017) In situ tissue engineering: endothelial growth patterns as a function of flow diverter design. J Neurointerv Surg 9:994–998. https://doi.org/10.1136/neurintsurg-2016-012669
Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ (2007) A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 38:2346–2352. https://doi.org/10.1161/STROKEAHA.106.479576
Ravindran K, Casabella AM, Cebral J, Brinjikji W, Kallmes DF, Kadirvel R (2020) Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery 86:S13–S19. https://doi.org/10.1093/neuros/nyz324
Mardia KV, Jupp PE (2000) Directional statistics. John Wiley & Sons, New York
Freed AD (2008) Anisotropy in hypoelastic soft-tissue mechanics, I: theory. J Mech Mater Struct 3:911–928. https://doi.org/10.2140/jomms.2008.3.911v
Cebral JR, Castro MA, Appanaboyina S, Putman CM, Millan D, Frangi AF (2005) Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans Med Imaging 24:457–467. https://doi.org/10.1109/TMI.2005.844159
Acknowledgments
The authors are grateful for support from National Institutes of Health under NINDS Grants 1R21NS08256 and 1R01NS097457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic and followed the NIH Guidelines for the Care and Use of Laboratory Animals.
Conflict of Interst
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Fiber dispersion model
The collagen component is treated as a collection of extensible fibers with a distribution of orientations. In general, a single collagen fiber will have a wavy conformation in reference configuration κ0, Fig. 2(d). Using local Cartesian coordinates with unit base vectors (e1, e2) (Fig. 2(c)), a direction is associated with undulated fiber m0 in reference configuration κ0 can be decomposed to m0 = cosθe1 + sinθe2. The undulated fiber is assumed to become load bearing when the material element in direction m0 has undergone a stretch of λa, Fig. 2(d). This activation stretch is an additional material property determined from average tortuosity of fibers. Then the fiber commences stretching when λf = λa . The true stretch of the fiber λt is then λt = λf/λa, where λf can be obtained from λf2 = C : m0 ⨂ m0.
The distribution of fibers was represented by probability density function (PDF) of fibers at arbitrary orientation m0 in the reference configuration. Motivated by the MPM data, only collagen fibers in 2D plane were considered. The PDF was represented by a bimodal von Mises distribution [67].
where a1 and a2 are symmetrical angles of two peaks, b1 and b2 are concentration parameters. I0(b) is the Bessel function of order zero defined by
and the distribution is normalized such that [68],
The four parameters (a1, a2, b1, b2) of Eq. (10) of each case were determined using maximum likelihood estimation by function MLE in Matlab (MathWorks Inc. Natick, MA) [51] based on collagen fiber orientations measured from the MPM stacks using ctFIRE. The parameters of ctFIRE were listed in Table 4, with more details on the functions found at https://loci.wisc.edu/software/ctfire.
The strain energy function of collagen in each layer is then constructed by integrating response of fibers over all directions [40, 50]. The strain energy function of collagen in each layer is of the form
where wfi is the strain energy of fibers in layer i, and defined as
where the Heaviside function is used to switch the fiber contribution off when under compression
Appendix 2: Determination of material parameters from uniaxial tension experiments
Material constants in the structurally motivated constitutive model were determined from multiphoton imaging and uniaxial mechanical testing. Assuming the tissue is incompressible, necessarily the deformation is isochoric with principal stretch values satisfying λ1λ2λ3 = 1. The corresponding deformation gradient tensor and Cauchy Green tensors are
where λi is the principal stretch in direction ei. Coordinate axis are chosen such that the loading direction is e2 and e3 is orthogonal to the sample. Then the principal components of the Cauchy stress tensor can be written as
By setting σ3 = 0, an expression for the Lagrange multiplier p can be obtained. An implicit relation between λ1 and λ2 can then be obtained by setting Eq. (18) to be zero. Using Eq. (17) along with this implicit relation and the experimental data (relation between σ1 and λ1), the parameters αi and βi can be obtained from minimizing the least-squares objective function. The interior-point optimization algorithm of FMINCON function in Matlab (R2017a, MathWorks Inc., Natick, MA) was used for this purpose.
Appendix 3: Summary of hemodynamics
Computational fluid dynamic studies were performed in case specific 3D reconstructed geometries of the aneurysm sac and surrounding vasculature to evaluate the consistency in the flow fields across the various models within and across the time points. Specific methods are summarized below and aneurysm metrics (including geometric, flow, and wall shear stress) for each case are given in Table 5.
All aneurysms had a relatively high aspect ratio (AR = height/neck diameter), ranging from 1.79 to 3.56, Table 5, with a bottle neck factor (BF) of 0.80 to 1.53. There was no statistically significant difference of AR or BF at different time points. Three cases, chosen to span a large range of AR and BF, are shown in Fig. 13. As a result of the high AR, the magnitude of wall shear stress was consistently low within the aneurysms, Table 5. For example, the time and space averaged magnitude of wall shear stress (TSAWSSM) had an average of 2.95 ± 1.29 dynes/cm2, five times less than the parent artery. The TSAWSSM was less than 7.0 dynes/cm2 for all cases.
Hemodynamic results for three representative cases. Each row represents one of three case chosen to span a large range of AR and BF values found in the rabbit models. (a)-(d) Streamlines at four different time points of the cardiac cycle. (e) Time averaged wall shear stress. (f) Maximum wall shear stress
The qualitative flow featues were similar across all cases, with two transient vortices present in the aneurysm sac, although the duration varied beween cases, Fig. 13. The inflow jet entered at the distal neck, then proceeded to impinge on the proximal wall, splitting into a slower vortex in the dome and a faster vortex near the neck. The flow generally exited through the proximal half of the neck plane. As a result of the concentrated inflow at the distal neck, the time average magnitude of wall shear stress was elevated at this location as exemplified in Fig. 13. The strength and location of jet impingement varied over the aneurysm wall and between cases. The effect of this local feature of the flow field on endothelial cell coverage, for example, is a topic of ongoing investigation.
Methods
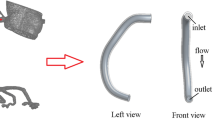
Of the 22 aneurysm models created in this study, 3DRA data for 14 cases were available for 3D digital reconstruction. Stereolithography (STL) data of the lumen were produced from the 3DRA data using the commercial software Mimics (Materialise NV, Leuven, Belgium). Finite element models of each lumen were created using a custom-developed software [69]. The finite element domain consisted of linear tetrahedral elements having an average element edge-length of 0.12 mm. The model domain included the ascending and descending aorta, the left subclavian (LS), left common carotid artery (LCCA), and the quadrification distal to the right subclavian arteries.
Blood was approximated as incompressible with constant kinematic viscosity of 3.33e-6 m2/s. The unsteady Navier-Stokes equations were solved over the computational domain assuming a rigid model of the vasculature so the no-slip condition as applied at the vessel wall. Animal specific doppler ultrasound (DUS) waveform data obtained proximal to the rabbit aneurysms in the brachiocephalic artery (BA) were used to set an inlet flow rate for the model. Briefly, the ultrasound velocity was taken as the peak velocity of a zeroeth order Womersley profile, from which the flow rate in the BA could be approximated. Separate DUS data were available in the LCCA and descending aorta and used to similarly approximate those flow rates. The flow split across outlets distal to the aneurysm were assumed to scale by Murray’s Law (i.e., the flow splits scales by the artery radius cubed). At each outlet, a Womersley velocity profile was then applied consistent with this flow division. Finally, the pressure was set via a traction free boundary condition at the ascending aortic inlet. The solution was discretized in time with timesteps of 0.001 s and obtained from FeFlo, the finite element solver described in [69].
Rights and permissions
About this article
Cite this article
Sang, C., Kallmes, D.F., Kadirvel, R. et al. Adaptive Remodeling in the Elastase-Induced Rabbit Aneurysms. Exp Mech 61, 263–283 (2021). https://doi.org/10.1007/s11340-020-00671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00671-9