Abstract
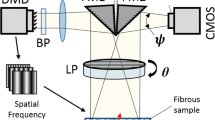
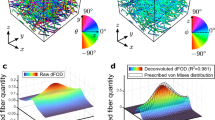
The underlying fiber architecture of soft tissues, like bat wing skin, plays an important role in the material’s overall mechanical behavior. The mesoscopic birefringent fiber architecture of the bat wing skin can be visualized directly under polarized light. This inherent property is the key to the new experimental technique developed in this work: polarized image correlation (PIC). PIC is a technique for determining full field material strains and fiber kinematics of mesoscopically resolved fibrous tissues during biaxial mechanical testing. Not only is the material birefringence used to determine fiber kinematics under finite deformations, but it is also used for image correlation and strain field computation. Pure translation tests performed with PIC verify the accuracy of the technique. A segmental image processing method was developed to solve an experimental issue of changing birefringent properties to construct accurate continuous deformation profiles. By integrating PIC with traditional digital image correlation, both surface and subsurface data give additional insight into through thickness tissue behavior. The PIC technique is applicable to semi-transparent tissues with birefringent mesosopic structures; incorporation of microscopy would resolve smaller fiber structures. Future work includes extending the techniques to three dimensions to analyze curved surfaces.









Similar content being viewed by others
Notes
To fully characterize these materials, independent control of three separate strain components (or stress components) is required [38]. This would require independent control of, for example, the in-plane shear strains in addition to the two axial strains (see Holzapfel and Ogden [38] for a comprehensive discussion of the experimental requirements for constitutive modeling.). For thin biological membranes, biaxial testing may be sufficient since 2D membrane models are often applied.
Clamping is another attachment option; however, in addition to potentially damaging the sample, clamping induces stress concentrations at the corners between clamps, effectively ‘stress-shielding’ the central region [39]. This stress-shielding phenomenon makes the tissue appear stiffer because the applied force is not fully transferred to the central region. In contrast, suturing methods allow the tissue to freely expand in the lateral direction, minimizing boundary effects [39].
References
Vaughan TA (1966) Morphology and flight characteristics of molossid bats. J Mammal 249–260
Hubel TY, Hristov NI, Swartz SM, Breuer KS (2010) Wake structure and wing kinematics: the flight of the lesser dog-faced fruit bat, Cynopterus brachyotis. J Exp Biol 213(20):3427–3440
Swartz SM, Groves MS, Kim HD, Walsh WR (1996) Mechanical properties of bat wing membrane skin. J Zool 239(2):357–378
Holbrook KA, Odland GF (1978) A collagen and elastic network in the wing of the bat. J Anat 126(Pt 1):21
Komatsu K, Chiba M (2001) Synchronous recording of load-deformation behaviour and polarized light-microscopic images of the rabbit incisor periodontal ligament during tensile loading. Arch Oral Biol 46(10):929–937
Tower TT, Neidert MR, Tranquillo RT (2002) Fiber alignment imaging during mechanical testing of soft tissues. Ann Biomed Eng 30(10):1221–1233
Komatsu K, Viidik A (1996) Changes in the fibre arrangement of the rat incisor periodontal ligament in relation to various loading levels in vitro. Achr Oral Biol 41(2):147–159
Tower TT, Tranquillo RT (2001) Alignment maps of tissues: II. Fast harmonic analysis for imaging. Biophys 81(5):2964–2971
Wang YN, Galiotis C, Bader DL (2000) Determination of molecular changes in soft tissues under strain using laser Raman microscopy. J Biomech 33(4):483–486
Voytik-Harbin SL, Roeder BA, Sturgis JE, Kokini K, Robinson JP (2003) Simultaneous mechanical loading and confocal reflection microscopy for three-dimensional microbiomechanical analysis of biomaterials and tissue constructs. Microsc Microanal 9(01):74–85
Hansen KA, Weiss JA, Barton JK (2002) Recruitment of tendon crimp with applied tensile strain. J Biomech Eng 124:72
Sacks MS, Smith DB, Hiester ED (1997) A small angle light scattering device for planar connective tissue microstructural analysis. Ann Biomed Eng 25(4):678–689
Gilbert TW, Sacks MS, Grashow JS, Savio LYW, Badylak SF, Chancello MB (2006) Fiber kinematics of small intestinal submucosa under biaxial and uniaxial stretch. J Biomech Eng 128:890
Sacks MS, Sun W (2003) Multiaxial mechanical behavior of biological materials. Annu Rev Biomed Eng 5:251
Lanir Y, Fung YC (1974) Two-dimensional mechanical properties of rabbit skin–I. Experimental system. J Biomech 7(1):29–34
Yin FCP, Strumpf RK, Chew PH, Zeger SL (1987) Quantification of the mechanical properties of noncontracting canine myocardium under simultaneous biaxial loading. J Biomech 20(6):577–589
Choi HS, Vito RP (1990) Two-dimensional stress–strain relationship for canine pericardium. J Biomech Eng 112(2):153–159
Billiar KL, Sacks MS (2000) Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—Part I: Experimental results. J Biomech Eng 122:23
Peters WH, Ranson WF (1982) Digital imaging techniques in experimental stress analysis. Opt Eng 21(3):213427
Sutton MA, Wolters WJ, Peters WH, Ranson WF, McNeill SR (1983) Determination of displacements using an improved digital correlation method. Image Vision Comput 1(3):133–139
Chu TC, Ranson WF, Sutton MA (1985) Applications of digital-image-correlation techniques to experimental mechanics. Exp Mech 25(3):232–244
Sacks MS (1999) A method for planar biaxial mechanical testing that includes in-plane shear. J Biomech Eng 121:551
Zhang D, Eggleton CD, Arola DD (2002) Evaluating the mechanical behavior of arterial tissue using digital image correlation. Exp Mech 42(4):409–416
Zhang D, Arola DD (2004) Applications of digital image correlation to biological tissues. J Biomed Opt 9:691
Hoc T, Henry L, Verdier M, Aubry D, Sedel L, Meunier A (2006) Effect of microstructure on the mechanical properties of Haversian cortical bone. Bone 38(4):466–474
Thompson MS, Schell H, Lienau J, Duda GN (2007) Digital image correlation: a technique for determining local mechanical conditions within early bone callus. Med Eng Phys 29(7):820–823
Sutton MA, Ke X, Lessner SM, Goldbach M, Yost M, Zhao F, Schreier HW (2008) Strain field measurements on mouse carotid arteries using microscopic three-dimensional digital image correlation. J Biomed Mater Res A 84(1):178–190
Gasser TC, Ogden RW, Holzapfel GA (2006) Hyperelastic modeling of arterial layers with distributed collagen fibre orientations. J R Soc Interface 3(6):15–35
Wang Y, Son S, Swartz SM, Goulbourne NC (2012) A mixed von Mises distribution for modeling soft biological tissues with two distributed fiber properties. Int J Solids Struct 49(21):2914–2923
Schreier HW, Braasch JR, Sutton MA (2000) Systematic errors in digital image correlation caused by intensity interpolation. Opt Eng 39:2915
Schreier HW, Sutton MA (2002) Systematic errors in digital image correlation due to undermatched subset shape functions. ExpMech 42(3):303–310
Lecompte D, Smits A, Bossuyt S, Sol H, Vantomme J, Van Hemelrijck D, Habraken AM (2006) Quality assessment of speckle patterns for digital image correlation. Opt Laser Eng 44(11):1132–1145
Pan B, Lu Z, Xie H (2010) Mean intensity gradient: an effective global parameter for quality assessment of the speckle patterns used in digital image correlation. Opt Laser Eng 48(4):469–477
Yaofeng S, Pang JHL (2007) Study of optimal subset size in digital image correlation of speckle pattern images. Opt Laser Eng 45(9):967–974
GOM optical measuring techniques (2009) Chapter D: Computation. In Aramis user manual - software, ver6.1. GOM, Braunschweig, Germany
Romhányi G (1983) The characteristic three-banded birefringence of ligamentum nuchae elastic fibres indicates ordered micellar texture. Histochem Cell Biol 77(1):133–139
Aaron BB, Gosline JM (1981) Elastin as a random–network elastomer: a mechanical and optical analysis of single elastin fibers. Biopolymers 20(6):1247–1260
Holzapfel GA, Ogden RW (2009) On planar biaxial tests for anisotropic nonlinearly elastic solids. A continuum mechanical framework. Math Mech Solids 14(5):474
Sun W, Sacks MS, Scott MJ (2005) Effects of boundary conditions on the estimation of the planar biaxial mechanical properties of soft tissues. J Biomech Eng 127:709
Acknowledgments
We acknowledge support of the Air Force Office of Scientific Research (Grant #F023809), the National Science Foundation (NSF IOS 1145549), and fellowship support from the Horace Rackham Graduate School of the University of Michigan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skulborstad, A.J., Wang, Y., Davidson, J.D. et al. Polarized Image Correlation for Large Deformation Fiber Kinematics. Exp Mech 53, 1405–1413 (2013). https://doi.org/10.1007/s11340-013-9751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-013-9751-4