Abstract
Background
The increasing of obesity is one of the main challenges of public health, with an incentive to the development of healthy life habits and practise of physical exercise. Among the physical exercises, the use of whole body vibration is highlighted, which can promote gain of muscle strength.
Aims
This study aimed atevaluating the effects that vibratory platform causes on the histomophometry and fiber types of the soleus muscle of Wistar rats with induced obesity by monosodium glutamate (MSG).
Methods
Thirty-two male Wistar rats were used, 16 of which were induced by obesity with intradermal injections of MSG, equally randomized into four groups: control (GC), control with intervention (GCP), obese (GO), and obese with intervention (GOP). At the 70 days, the training on vibratory platform was started adapted to a frequency of 60 Hz and amplitude of 2 mm, performed 3 times a week, with a duration of 10 min, during 8 consecutive weeks. At 130 days, the animals were weighed and the nasoanal length was measured; then they were euthanized and the soleus muscles were collected and processed for analysis. Data were statistically analyzed about the homogeneity of variances by the Bartlett’s test, about the normality by Shapiro–Wilk’s test, then ANOVA of two factors and Tukey-HSD follow-up tests, adopting the significance level of 5%.
Results
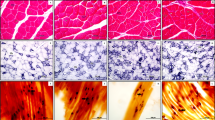
Morphometrically, the obese groups presented muscle hypotrophy (p < 0.01) and the training caused increase in the fibers (p = 0.03), increase in the number of nuclei (p < 0.01), and decrease of connective tissue (p < 0.01). Meanwhile, there was no distinction among the muscle fiber types after the training on the vibratory platform, which is composed of oxidative fibers.
Conclusion
The training on the vibratory platform induced beneficial effects in the muscle tissue of obese rats; however, both obesity and training did not influence on the fiber type of the soleus muscle.



Similar content being viewed by others
Abbreviations
- BW:
-
Body weight
- CEUA:
-
Animal Ethics Committee
- GC:
-
Control group
- GCP:
-
Control group + vibratory platform
- GO:
-
Obese group
- GOP:
-
Obese group + vibratory platform
- LABEF:
-
Laboratory of structural and functional biology
- LELRF:
-
Laboratory of physical injury and physiotherapeutic resources
- MSG:
-
Monosodium glutamate
- NL:
-
Nasoanal length
- NCDs:
-
Chronic non-communicable diseases
- OS:
-
Obesity sarcopenic
- UNIOESTE:
-
Universidade Estadual do Oeste do Paraná
References
WHO. World Health Organization. Disponível em: https://www.who.int/topics/obesity/en/. Acesso em: 05 jan. 2019.
Tomlinson DJ (2016) The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 17(3):467–483. https://doi.org/10.1007/s10522-015-9626-4
Rahemi H, Nigam N, Wakeling JM (2015) The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface 12(109):365–372. https://doi.org/10.1098/rsif.2015.0365
Talbot J, Maves L (2016) Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip Rev Dev Biol 5:518–534. https://doi.org/10.1002/wdev.230
Almeida L, Ramos K, Randow R, Guerra V (2017) Estratégias e desafios da gestão da atenção primária à saúde no controle e prevenção da obesidade. Rev Gestão Saúde 8(1):114–139
Schuh DS, Goulart MR, Barbiero SM, Sica CD, Borges R et al (2017) Escola saudável é mais feliz: design e protocolo de um ensaio clinico randomizado desenvolvido para prevenir o ganho de peso em crianças. Arq Bras Cardiol 108(6):501–507. https://doi.org/10.5935/abc.20170072
Frontera WR, Ochala J (2015) Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 96(3):183–195. https://doi.org/10.1007/s00223-014-9915-y
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184. https://doi.org/10.1016/j.cmet.2012.12.012
Anwer S, Alghadir A, Zafar H, Al-Eisa E (2016) Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: a systematic review and meta analysis. Physiotherapy 102(2):145–151. https://doi.org/10.1016/j.physio.2015.10.004
Cerciello S, Rossi S, Visonà E, Corona K, Oliva F (2016) Clinical applications of vibration therapy in orthopaedic practice. Muscles Ligaments Tendons J 6(1):147–156. https://doi.org/10.11138/mltj/2016.6.1.147
Corbiere TF, Weinheimer-Haus EM, Judex S, Koh TJ (2018) Low-intensity vibration improves muscle healing in a mouse model of laceration injury. J Funct Morphol Kinesiol 3(1):1. https://doi.org/10.3390/jfmk3010001
Kaneguchi A, Ozawa J, Kawamata S, Kurose T, Yamaoka K (2014) Intermittent whole-body vibration attenuates a reduction in the number of the capillaries in unloaded rat skeletal muscle. BMC Musculoskelet Disord 15(315):01–9. https://doi.org/10.1186/1471-2474-15-315
Komrakova M, Sehmisch S, Tezval M, Ammon J, Lieberwirth P, Sauerhoff C et al (2013) Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int 92(6):509–520. https://doi.org/10.1007/s00223-013-9706-x
Rubio-Arias JÁ, Ramos-Campo DJ, Esteban P, Martínez F, Jiménez JF (2018) Effect of 6-weeks WBVT on the behavior of the lower limb muscle fibres during vertical jumping. J Sports Sci 36(4):398–406. https://doi.org/10.1080/02640414.2017.1309059
Jacobson BH, Monaghan TP, Sellers JH, Conchola EC, Pope ZK, Glass RG (2017) Acute effect of biomechanical muscle stimulation on the counter-movement vertical jump power and velocity in division i football players. J Strength Cond Res 31(5):1259–1264. https://doi.org/10.1519/JSC.0000000000001136
Cantelli KR, Soares GM, Ribeiro RA, Balbo SL, Lubaczeuski C, Boschero AC, Araújo ACF, Bonfleur ML (2017) Duodenal-jejunal bypass normalizes pancreatic islet proliferation rate and function but not hepatic steatosis in hypothalamic obese rats. Braz J Med Biol Res 50(5):e5858. https://doi.org/10.1590/1414-431X20175858
Wang J, Leung KS, Chow SKH, Cheunga WH (2017) Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J Orthop Translat 10:94–101. https://doi.org/10.1016/j.jot.2017.05.006
Pertti AL, Kakihata CMM, Wutzke MLS, Torrejais MM, Ribeiro LFC, Bertolini GRFB (2019) Effects of mechanical vibration in neuromuscular junctions and fiber type of the soleus muscle of oophorectomized wistar rats. Rev Bras Ortop 54(5):572–578. https://doi.org/10.1055/s-0039-1697016
Thomas GA, Kraemer WJ, Comstock BA, Dunn-Lewis C, Maresh CM, Volek JS (2013) Obesity, growth hormone and exercise. Sports Med 43(9):839–849. https://doi.org/10.1007/s40279-013-0064-7
Bollinger L (2017) Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture 56:100–107. https://doi.org/10.1016/j.gaitpost.2017.05.003
Tamilarasan KP, Temmel H, Das SK, Zoughbi WA, Schauer S, Vesely PW et al (2012) Skeletal muscle damage and impaired regeneration due to LPL-mediated lipotoxicity. Cell Death Dis 3(354):01–8. https://doi.org/10.1038/cddis.2012.91
Matsakas A, Prosdocimo DA, Mitchell R, Collins-Hooper H, Giallourou N, Swann JR et al (2015) Investigating mechanisms underpinning the detrimental impact of a high-fat diet in the developing and adult hypermuscular myostatin null mouse. Skelet Muscle 5(38):01–21. https://doi.org/10.1186/s13395-015-0063-5
Akhmedov D, Berdeaux R (2013) The effects of obesity on skeletal muscle regeneration. Front Physiol 4(371):01–12. https://doi.org/10.3389/fphys.2013.00371
Łochyński D, Kaczmarek D, Redowicz MJ, Celichwski J (2013) Long-term effects of whole-body vibration on motor unit contractile function and myosin heavy chain composition in the rat medial gastrocnemius. J Musculoskelet Neuronal Interact 13(4):430–441
Krajnak K, Riley DA, Wu J, Mcdowell T, Welcome DE, Xu XS et al (2012) Frequency-dependent effects of vibration on physiological systems: experiments with animals and other human surrogates. Ind Health 50(5):343–353
Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA (2005) Vibration-induced disruption of retrograde axoplasmic transport in peripheral nerve. Muscle Nerve 32:521–526. https://doi.org/10.1002/mus.20379
Córdova A, Navas FJ (2000) Os radicais livres e o dano muscular produzido pelo exercício: papel dos antioxidants. Rev Bras Med Esporte 6(5):204–208. https://doi.org/10.1590/S1517-86922000000500006
Waugh S, Kashon ML, Li S, Miller GR, Johnson C, Krajnak K (2016) Transcriptional pathways altered in response to vibration in a model of hand-arm vibration syndrome. J Occup Environ Med 58(4):344–350. https://doi.org/10.1097/JOM.0000000000000705
Barbe MF, Barr AE (2006) Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun 20(5):423–429. https://doi.org/10.1016/j.bbi.2006.03.001
Glazebrook MA, Wright JR Jr, Langman M, Stanish WD, Lee JM (2008) Histological analysis of achilles tendons in an overuse rat model. J Orthop Res 26(6):840–846. https://doi.org/10.1002/jor.20546
Parks AN, McFaline-Figueroa J, Coogan A, Poe-Yamagata E, Guldberg RE, Platt MO et al (2017) Supraspinatus tendon overuse results in degenerative changes to tendon insertion region and adjacent humeral cartilage in a rat model. J Orthop Res 35(9):1910–1918
Chen YM, Lee HC, Chen MT, Huang CC, Chen WC (2018) Dehydroepiandrosterone supplementation combined with Weight-Loading Whole-Body Vibration Training (WWBV) affects exercise performance and muscle glycogen storage in middle-aged C57BL/6 mice. Int J Med Sci 15(6):564–573. https://doi.org/10.7150/ijms.233522018
Sitnick M, Bodine SC, Rutledge JC (2009) Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol 587(23):5753–5765. https://doi.org/10.1113/jphysiol.2009
Gavin TP, Stallings HW, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA et al (2005) Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98(1):315–321. https://doi.org/10.1152/japplphysiol.00353.2004
Yan Z, Lira VA, Greene NP (2012) Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40(3):159–164. https://doi.org/10.1097/JES.0b013e3182575599
Tidball JG, Villalta SG (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298:1173–1187. https://doi.org/10.1152/ajpregu.00735.2009
Gundersen K (2016) Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol 219(2):235–242. https://doi.org/10.1242/jeb.124495
Baoge L, Steen EVD, Rimbaut S, Philips N, Witvrouw E, Almqvist KF et al (2012) Treatment of skeletalmuscle injury: a review. Int Sch Res Netw 2012:01–7. https://doi.org/10.5402/2012/689012
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. https://doi.org/10.1016/j.cell.2012.03.017
Bucci M, Vinagre EC, Campos GER, Curi R, Pithon-Curi TC (2005) Efeitos do treinamento concomitante hipertrofia e endurance no músculo esquelético. R Bras Ci Mov 13(1):17–28
Ramos LA, Carvalho RT, Abdalla RJ, Ingham SJM (2015) Surgical treatment for muscle injuries. Curr Rev Musculoskelet Med 8(2):188–192. https://doi.org/10.1007/s12178-015-9272-0
Gillies AR, Lieber RL (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44(3):318–331. https://doi.org/10.1002/mus.22094
Wright T, Langley-Evans SC, Voigt JP (2011) The impact of maternal cafeteria diet on anxiety-related behaviour and exploration in the offspring. Physiol Behav 103(2):164–172. https://doi.org/10.1016/j.physbeh.2011.01.008
Serrano AL, Muñoz-Cánoves P (2010) Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316:3050–3058. https://doi.org/10.1016/j.yexcr.2010.05.035
Rocha WA, Gobbi GA, Araujo VF, Santuzzi CH (2010) Muscle morphological changes in response to passive stretching in an animal model of prolonged immobilization of hind limb. Rev Bras Med Esporte 16(6):450–454. https://doi.org/10.1590/S1517-86922010000600011
Santana Junior A, Debastiani JC, Buratti P, Peretti AL, Kunz RI, Brancalhão RMC et al (2018) Sericina e natação sobre parâmetros histomorfométricos de músculo plantar desnervado de ratos Wistar. Einstein 16(1):01–6. https://doi.org/10.1590/S1679-45082018AO4137
Haizlip KM, Harrison BC, Leinwand LA (2015) Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology 30(1):30–39. https://doi.org/10.1152/physiol.00024.2014
Camargo Filho JCS, Vanderlei LCM, Camargo RCT, Oliveira DAR, Oliveira Júnior SA, Pai VD (2005) Análise histológica, histoquímica e morfométrica do músculo sóleo de ratos submetidos a treinamento físico em esteira rolante. Arq Ciênc Saúde 12(3):196–199
Deschenes MR, Adan MA, Kapral MC, Kressin KA, Leathrum CM, Seo A et al (2018) Neuromuscular adaptability of male and female rats to muscle unloading. J Neurosci Res 96(2):284–296. https://doi.org/10.1002/jnr.24129
Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K et al (2017) Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep 5(7):01–12. https://doi.org/10.14814/phy2.13250
Adachi T, Kikuchi N, Yasuda K, Anahara R, Gu N, Matsunaga T et al (2007) Fibre type distribution and gene expression levels of both succinate dehydrogenase and peroxisome proliferator-activated receptor-γ coactivator-1α of fibres in the soleus muscle of Zucker diabetic fatty rats. Exp Physiol 92(2):449–455. https://doi.org/10.1113/expphysiol.2006.035451
Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R et al (2006) Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29(4):895–900
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors report no conflicts of interest.
Ethics approval
The research Project was approved by the Animal Ethics Committee (CEUA) from UNIOESTE (Number 08/18).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boaretto, M.L., de Andrade, B.Z., Maciel, J.I.H.N. et al. Effects of vibratory platform training on the histomorphometric parameters of the soleus muscle in obese Wistar rats. Sport Sci Health 16, 501–510 (2020). https://doi.org/10.1007/s11332-020-00632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-020-00632-8