Abstract
Background
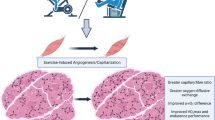
Diabetes mellitus (Di) induces the inflammation and destruction of remodeling in cardiovascular environment. Exercise with increase in levels of cardiokines such as FSTL-1 and angiogenesis-inducing factors has cardioprotective effect in some heart diseases. Thus, the purpose of this study was to investigate the effects of moderate-intensity aerobic exercise and insulin injection on levels of cardiac FSTL-1, NDNF, and VEGF in diabetic rats.
Methods
To test the hypothesis that FSTL-1 may be a molecular link between exercise and improved cardiac remodeling and angiogenesis in diabetic heart, we subjected diabetic rats, induced by intraperitoneal injection of STZ (55 mg/kg, freshly dissolved in 0.4 ml citrate buffer, pH 4.5). The rats were randomly divided into four groups (n = 6 in each group): control Di (without exercise and without insulin), insulin Di (without exercise and with insulin), exercise Di (with exercise and without insulin), and insulin and exercise Di (with exercise and with insulin), for 6 weeks and examined the relevance of FSTL-1, NDNF, and VEGF to exercise-mediated cardiac effects. The 6-week exercise training intervention involved 30 min of moderate-intensity running on a treadmill once daily (5 d/w). Measurement levels of cardiac FSTL-1, NDNF, and VEGF were performed an ELISA kit. Representative image of myocardium stained with H&E. Data were analyzed by two-way ANOVA and Pearson correlation coefficient.
Results
The results of this study show that exercise or insulin injection alone has a significant effect on FSTL-1 and NDNF (p ≤ 0.05). Also, there is a significant interaction between exercise and insulin in FSTL-1 (P = 0.001, F = 61.612), and NDNF (P = 0.001, F = 29.414) and VEGF (P = 0.001, F = 29.414).
Conclusion
The results of this study showed that insulin and exercise Di group had better effects on NDNF and VEGF in diabetic animal. Also, FSTL-1 changes and functions in the heart of diabetic rats were different from other pathology such as MI sample. However, in this regard and investigating these factors in the heart of the diabetic sample, more studies are needed.





Similar content being viewed by others
References
Association AD (2019) Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42(1):13–28
Pappachan JM et al (2013) Diabetic cardiomyopathy: pathophysiology, diagnostic evaluation and management. World J Diabetes 4(5):177
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115(25):3213–3223
Muralidaran Y, Viswanathan P (2015) Diabetic cardiomyopathy: A new perspective of mechanistic approach. J Diabetes Metab 6(10):1–15
Shimano M, Ouchi N, Walsh K (2012) Cardiokines: recent progress in elucidating the cardiac secretome. Circulation 126(21):e327–e332
Hayakawa S et al (2015) Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model. J Am Soc Nephrol 26(3):636–646
Kawabata D et al (2004) Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthr Rheum 50(2):660–668
Miyamae T et al (2006) Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol 177(7):4758–4762
Fan N et al (2013) Follistatin-like 1: a potential mediator of inflammation in obesity. Mediat Inflamm. https://doi.org/10.1155/2013/752519
Wei K et al (2015) Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525(7570):479
Oshima Y et al (2008) Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117(24):3099
Lara-Pezzi E et al (2008) Expression of follistatin-related genes is altered in heart failure. Endocrinology 149(11):5822–5827
Ouchi N et al (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 283(47):32802–32811
Ouchi N et al (2016) Protective roles of adipocytokines and myokines in cardiovascular disease. Circ J. https://doi.org/10.1253/circj.CJ-16-0663
Kuang X-L et al (2010) Spatio-temporal expression of a novel neuron-derived neurotrophic factor (NDNF) in mouse brains during development. BMC Neurosci 11(1):137
Ohashi K et al (2014) Neuron-derived neurotrophic factor functions as a novel modulator that enhances endothelial cell function and revascularization processes. J Biol Chem 289(20):14132–14144
Joki Y et al (2015) Neuron-derived neurotrophic factor ameliorates adverse cardiac remodeling after experimental myocardial infarction. Circulation 8(2):342–351
Wu Y-S et al (2018) The role of cardiokines in heart diseases: beneficial or detrimental? BioMed Res Int. https://doi.org/10.1155/2018/8207058
Shirvani H et al (2019) Short-term effect of low-, moderate-, and high-intensity exercise training on cerebral dopamine neurotrophic factor (CDNF) and oxidative stress biomarkers in brain male Wistar rats. Comp Clin Pathol 28(2):369–376
Hoeben A et al (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56(4):549–580
Chou E et al (2002) Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105(3):373–379
Aiello LP, Wong J-S (2000) Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int 58:S113–S119
Shirvani H, Aslani J (2017) The effects of high-intensity interval training vs. moderate-intensity continuous training on serum irisin and expression of skeletal muscle PGC-1α gene in male rats. Tehran Univ Med J TUMS Publ 75(7):513–520
Shirvani H, Rahmati-Ahmadabad S (2018) The combined effect of high intensity interval training and flaxseed oil supplement on cardioprotection: by UCP2, UCP3 and eNOS mRNA expression. J Mazandaran Univ Med Sci 28(160):8–18
TaheriChadorneshin H, Rostamkhani F, Shirvani H (2019) Long-term effects of sprint interval training on expression of cardiac genes involved in energy efficiency. Sport Sci Health 15(1):59–63
Mohammad Rahimi G, Attarzadeh Hosseini S (2014) The effect of aerobic training and diet on lipid profile and liver enzymes in obese women with type II diabetes. Daneshvar Med 21:41–50
Mohammad Rahimi G, Hosseini SRA (2016) Effect of aerobic training and diet on insulin resistance and quality of life in type II diabetic patients. Horiz Med Sci 22(1):57–64
Shirvani H, Arabzadeh E, Akbari J (2019) The short-term effect of caffeine supplementation on immune-endocrine responses to acute intensive exercise. Sci Sports. https://doi.org/10.1016/j.scispo.2019.07.003
Shirvani H, Sobhani V (2016) The effect of a period of selected aerobic training on the response of thyroid and cortisol hormones to exhaustive exercise in women. J Mil Med 18(3):253–261
Tayebi SM et al (2019) Plasma retinol-binding protein-4 and tumor necrosis factor-α are reduced in postmenopausal women after combination of different intensities of circuit resistance training and Zataria supplementation. Sport Sci Health 15:551–558
Shirvani H (2018) Short-term effects of supplementation of Coenzyme Q10 on humoral immune response to high intensity intermittent exercise in male soccer players. Koomesh 20(1):122–130
Rahmati-Ahmadabad S et al (2018) The effects of high-intensity interval training on reverse cholesterol transport elements: a way of cardiovascular protection against atherosclerosis. Life Sci 209:377–382
Shirvani H, Rahmati-Ahmadabad S (2019) Irisin interaction with adipose tissue secretions by exercise training and flaxseed oil supplement. Lipids Health Dis 18(1):15
Shirvani H et al (2018) Changes in monocarboxylate transporter 1 and p53 gene expression by aerobic interval training in the experimental colon carcinoma of mouse. Tehran Univ Med J TUMS Publ 76(5):313–320
Mirdar S, Arabzadeh E, Hamidian G (2015) Effects of two and three weeks of tapering on lower respiratory tract in the maturing rat. Koomesh 16(3):366–375
Kilkenny C et al (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Grant CW et al (2012) Development of standardized insulin treatment protocols for spontaneous rodent models of type 1 diabetes. Comp Med 62(5):381–390
Yoon H et al (2015) Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol 267:107–114
Sobhani V et al (2018) High-intensity interval training-induced inflammation and airway narrowing of the lung parenchyma in male maturing rats. Comp Clin Pathol 27(3):577–582
Rahmati-Ahmadabad S, Shirvani H, Sobhani V (2018) Long term effect of high intensity interval training and flaxseed oil supplementation on the expression of genes involved in reverse cholesterol transport in male rats. J Med Plants 4(64):59–75
Høydal MA et al (2007) Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14(6):753–760
Arabzadeh E, Mirdar S, Fathi Z (2015) Measurement of levels of lung HIF-1α protein in response to tapering for 14-and 21-day with Nigella sativa supplementation in maturing rat, with histological study. Sport Sci Health 11(2):195–202
Alizadeh AM et al (2018) Oxytocin mediates the beneficial effects of the exercise training on breast cancer. Exp Physiol 103(2):222–235
Mirdar S et al (2019) The effects of tapering with and without ethanolic extract of Nigella sativa on hypoxia inducible factor-1α and exercise-induced bronchial changes. J Mil Med 21(2):131–141
de Bem GF et al (2018) Antidiabetic effect of Euterpe oleracea Mart.(açai) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: a positive interaction. PLoS ONE 13(6):e0199207
Prajapati HB (2017) Role of aerobic exercise as an antidiabetic therapy in type2 diabetes mellitus: a pilot study. Int J Ther Rehabil Res 6(1):76
Arabzadeh E, Mirdar S, Moradiani H (2016) Nigella sativa supplementation attenuates exercise-induced bronchoconstriction in the maturing rat: a histometric and histologic study. Comp Clin Pathol 25(1):1–5
Shirvani H et al (2019) Eccentric resistance training and β-hydroxy-β-methylbutyrate free acid affects muscle PGC-1α expression and serum irisin, nesfatin-1 and resistin in rats. J Exp Biol. https://doi.org/10.1242/jeb.198424
Shirvani H, Rahimi M, Rostamkhani F (2015) Effect of a karate competition on indicators of inflammation and muscle tissue injury in soldier's karate-Ka. J Mil Med 17(3):137–143
Xi Y, Gong D-W, Tian Z (2016) FSTL1 as a potential mediator of exercise-induced cardioprotection in post-myocardial infarction rats. Sci Rep 6:32424
Widera C et al (2009) Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem 55(10):1794–1800
Rahmati-Ahmadabad S et al (2019) Effects of exercise on reverse cholesterol transport: a systemized narrative review of animal studies. Life Sci. https://doi.org/10.1016/j.lfs.2019.03.058
Goudarzian M et al (2017) Effects of whole body vibration training and mental training on mobility, neuromuscular performance, and muscle strength in older men. J Exerc Rehabi 13(5):573
Acknowledgements
The authors wish to thank Department of Exercise Physiology and Corrective Exercises, Faculty of Sport Sciences, Urmia University for scientific supports. Moreover, the Authors would like to thank RASTA Lab.Co for laboratory supports.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All procedures performed in studies involving animal participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki. All animal studies were approved by the Ethical Committee supervising procedures on experimental animals at Urmia University of Medical Sciences (Ethical cod# IR.UMSU.REC.1397.065).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arabzadeh, E., Samadian, Z., Tofighi, A. et al. Alteration of follistatin-like 1, neuron-derived neurotrophic factor, and vascular endothelial growth factor in diabetic cardiac muscle after moderate-intensity aerobic exercise with insulin. Sport Sci Health 16, 491–499 (2020). https://doi.org/10.1007/s11332-020-00631-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-020-00631-9