Abstract
Purpose
Home sleep testing devices are being widely used in diagnosis/screening for obstructive sleep apnea (OSA). We examined differences in OSA metrics obtained from two devices with divergent home monitoring strategies, the Apnea Risk Evaluation System (ARES™, multiple signals plus forehead reflectance oximetry) and the Nonin WristOx2™ (single channel finger transmission pulse oximeter), compared to differences from night-night variability of OSA.
Methods
One hundred fifty-two male/26 female subjects (BMI = 30.3 ± 5.6 kg/m2, age = 52.5 ± 8.9 years) were recruited without regard to OSA symptoms and simultaneously wore both ARES™ and Nonin WristOx2™ for two nights (n = 351 nights). Automated analysis of the WristOx2 yielded oxygen desaturation index (ODIOx2, ≥4% O2 dips/h), and automated analysis with manual editing of ARES™ yielded AHI4ARES (apneas + hypopneas with ≥4% O2 dips/h) and RDIARES (apneas + hypopneas with ≥4% O2 dips/h or arousal surrogates). Baseline awake oxygen saturation, percent time < 90% O2 saturation (%time < 90%O2Sat), and O2 signal loss were compared between the two methods.
Results
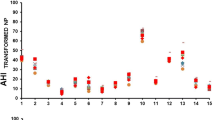
Correlation between AHI4ARES and ODIOx2 was high (ICC = 0.9, 95% CI = 0.87–0.92, p < 0.001, bias ± SD = 0.7 ± 6.1 events/h). Agreement values for OSA diagnosis (77–85%) between devices were similar to those seen from night-to-night variability of OSA using a single device. Awake baseline O2 saturation was significantly higher in the ARES™ (96.2 ± 1.6%) than WristOx2™ (92.2 ± 2.1%, p < 0.01). There was a significantly lower %time < 90%O2Sat reported by the ARES™ compared to WristOx2 (median (IQR) 0.5 (0.0, 2.6) vs. 2.1 (0.3, 9.7), p < 0.001), and the correlation was low (ICC = 0.2).
Conclusions
OSA severity metrics predominantly dependent on change in oxygen saturation and metrics used in diagnosis of OSA (AHI4 and ODI) correlated well across devices tested. However, differences in cumulative oxygen desaturation measures (i.e., %time < 90%O2Sat) between the devices suggest that caution is needed when interpreting this metric particularly in populations likely to have significant hypoxia.




Similar content being viewed by others
References
Hang LW, Wang HL, Chen JH, Hsu JC, Lin HH, Chung WS et al (2015) Validation of overnight oximetry to diagnose patients with moderate to severe obstructive sleep apnea. BMC Pulm Med 15:24
Chai-Coetzer CL, Antic NA, Hamilton GS, McArdle N, Wong K, Yee BJ et al (2017) Physician decision making and clinical outcomes with laboratory polysomnography or limited-channel sleep studies for obstructive sleep apnea: a randomized trial. Ann Intern Med 166(5):332–340
Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ et al (2007) Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 3(7):737–747
Ayappa I, Norman RG, Seelall V, Rapoport DM (2008) Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med 4(1):26–37
Vazquez JC, Tsai WH, Flemons WW, Masuda A, Brant R, Hajduk E et al (2000) Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax 55(4):302–307
Zou D, Grote L, Peker Y, Lindblad U, Hedner J (2006) Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep 29(3):367–374
Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D et al (2005) Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest 128(4):2166–2175
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K et al (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13(3):479–504
Kisch-Wedel H, Bernreuter P, Kemming G, Albert M, Zwissler B (2009) Does the estimation of light attenuation in tissue increase the accuracy of reflectance pulse oximetry at low oxygen saturations in vivo? IEEE Trans Biomed Eng 56(9):2271–2279
Zafar S, Ayappa I, Norman RG, Krieger AC, Walsleben JA, Rapoport DM (2005) Choice of oximeter affects apnea-hypopnea index. Chest 127(1):80–88
Davila DG, Richards KC, Marshall BL, O’Sullivan PS, Osbahr LA, Huddleston RB et al (2003) Oximeter’s acquisition parameter influences the profile of respiratory disturbances. Sleep 26(1):91–95
Cross TJ, Keller-Ross M, Issa A, Wentz R, Taylor B, Johnson B (2015) The impact of averaging window length on the “desaturation indexes during overnight pulse oximetry at high-altitude”. Sleep 38(8):1331–1334
Stepnowsky C, Levendowski D, Popovic D, Ayappa I, Rapoport DM (2013) Scoring accuracy of automated sleep staging from a bipolar electroocular recording compared to manual scoring by multiple raters. Sleep Med 14(11):1199–1207
Flemons WW, Littner MR (2003) Measuring agreement between diagnostic devices. Chest 124(4):1535–1542
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Levendowski D, Steward D, Woodson BT, Olmstead R, Popovic D, Westbrook P (2009) The impact of obstructive sleep apnea variability measured in-lab versus in-home on sample size calculations. Int Arch Med 2(1):2
Newell J, Mairesse O, Verbanck P, Neu D (2012) Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res 200(2–3):795–801
Le Bon O, Hoffmann G, Tecco J, Staner L, Noseda A, Pelc I et al (2000) Mild to moderate sleep respiratory events: one negative night may not be enough. Chest 118(2):353–359
Prasad B, Usmani S, Steffen AD, Van Dongen HP, Pack FM, Strakovsky I et al (2016) Short-term variability in apnea-hypopnea index during extended home portable monitoring. J Clin Sleep Med 12(6):855–863
Nigro CA, Aimaretti S, Gonzalez S, Rhodius E (2009) Validation of the WristOx 3100 oximeter for the diagnosis of sleep apnea/hypopnea syndrome. Sleep Breath 13(2):127–136
Romem A, Romem A, Koldobskiy D, Scharf SM (2014) Diagnosis of obstructive sleep apnea using pulse oximeter derived photoplethysmographic signals. J Clin Sleep Med 10(3):285–290
Chiner E, Signes-Costa J, Arriero JM, Marco J, Fuentes I, Sergado A (1999) Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax 54(11):968–971
Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA et al (2011) Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med 7(5):531–548
Dawson A, Loving RT, Gordon RM, Abel SL, Loewy D, Kripke DF et al (2015) Type III home sleep testing versus pulse oximetry: is the respiratory disturbance index better than the oxygen desaturation index to predict the apnoea-hypopnoea index measured during laboratory polysomnography? BMJ Open 5(6):e007956
Yamaura K, Nanishi N, Higashi M, Hoka S (2014) Effects of thermoregulatory vasoconstriction on pulse hemoglobin measurements using a co-oximeter in patients undergoing surgery. J Clin Anesth 26(8):643–647
The Report of an American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22(5):667–689
Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM (2001) Classification of sleep-disordered breathing. Am J Respir Crit Care Med 163(2):398–405
Masdeu MJ, Ayappa I, Hwang D, Mooney AM, Rapoport DM (2010) Impact of clinical assessment on use of data from unattended limited monitoring as opposed to full-in lab PSG in sleep disordered breathing. J Clin Sleep Med 6(1):51–58
Ayappa I, Rapaport BS, Norman RG, Rapoport DM (2005) Immediate consequences of respiratory events in sleep disordered breathing. Sleep Med 6(2):123–130
Acknowledgements
This study was supported by NIOSH/CDC U01OH010415 and NIH K24 grant HL109156.
Funding
This study was funded by NIOSH/CDC U01OH010415 and NIH K24 grant HL109156.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
TG, AT, SA, AP, and KB have no conflicts of interest to disclose.
DMR has received support for research from the industry in the past 24 months: grants from Fisher & Paykel Healthcare and speaking and consulting engagements for Fisher & Paykel Healthcare. DMR holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSA and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher & Paykel Healthcare, Advanced Brain Monitoring, and Sefam Medical.
JS has received support for speaker training from Merck Pharmaceuticals.
IA has received support for research from the industry in the past 24 months: grants from Fisher & Paykel Healthcare. IA holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSA and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring.
Additional information
Work performed at NYU Sleep Disorders Center and Rutgers Robert Wood Johnson Medical School
Rights and permissions
About this article
Cite this article
Gumb, T., Twumasi, A., Alimokhtari, S. et al. Comparison of two home sleep testing devices with different strategies for diagnosis of OSA. Sleep Breath 22, 139–147 (2018). https://doi.org/10.1007/s11325-017-1547-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1547-9