Abstract
Purpose
Fluorescence-guided surgery using a tumor-specific antibody-dye conjugate is useful in various cancer types. Fluorescence imaging is a valuable tool both intraoperatively and postoperatively for ex vivo imaging. The color of inks used for tumor specimens during ex vivo specimen processing in pathology is an important consideration for fluorescence imaging since the absorption/emission of the dyes may interfere with the fluorescent dye. This study assesses suitable ink colors for use specifically with IRDye800CW fluorescence imaging.
Procedures
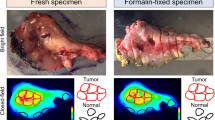
Eight tissue-marking inks or dyes (TMDs) commonly used for pathological evaluation were assessed. Agarose tissue-mimicking phantoms containing Panitumumab-IRDye800CW were used as an initial model. Mean fluorescence intensity was measured at 800 nm using both Pearl Trilogy as a closed-field fluorescence imaging system and pde-neo II as an open-field fluorescence imaging system before and after TMD application. An in vivo mouse xenograft model using the human head and neck squamous cell carcinoma FaDu cell line was then used in conjunction with TMDs.
Results
The retained IRDye800CW fluorescence on Pearl Trilogy was as follows: yellow at 91.0 ± 4.5%, red at 90.6 ± 2.7%, orange at 88.2 ± 2.2%, violet at 56.6 ± 1.1%, lime at 40.9 ± 1.8%, green at 19.3 ± 2.8%, black at 13.3 ± 0.6%, and blue at 8.1 ± 0.2%. The retained IRDye800CW fluorescence on pde-neo II was as follows: yellow at 86.5 ± 6.4%, red at 77.0 ± 6.2%, orange at 76.9 ± 2.8%, lime at 72.5 ± 9.5%, violet at 59.7 ± 0.4%, green at 30.1 ± 6.9%, black at 17.0 ± 2.7%, and blue at 6.7 ± 1.7%. The retained IRDye800CW fluorescence in yellow and blue TMDs was 42.1 ± 14.9% and 0.2 ± 0.2%, respectively in the mouse experiment (p = 0.039).
Conclusion
Yellow, red, and orange TMDs should be used, and blue and black TMDs should be avoided for evaluating tumor specimens through fluorescence imaging using IRDye800CW.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhang RR, Schroeder AB, Grudzinski JJ et al (2017) Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol 14:347–364
Lauwerends LJ, van Driel P, Baatenburg de Jong RJ et al (2021) Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol 22:e186–e195
Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL (2019) Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol 20:e354–e367
Lamberts LE, Koch M, de Jong JS et al (2017) Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res 23:2730–2741
Harlaar NJ, Koller M, de Jongh SJ et al (2016) Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol Hepatol 1:283–290
de Jongh SJ, Tjalma JJJ, Koller M et al (2020) Back-table fluorescence-guided imaging for circumferential resection margin evaluation using bevacizumab-800CW in patients with locally advanced rectal cancer. J Nucl Med 61:655–661
Mulder BGS, Koller M, Duiker EW et al (2023) Intraoperative molecular fluorescence imaging of pancreatic cancer by targeting vascular endothelial growth factor: a multicenter feasibility dose-escalation study. J Nucl Med 64:82–89
Rosenthal EL, Warram JM, de Boer E et al (2015) Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 21:3658–3666
Moore LS, Rosenthal EL, Chung TK et al (2017) Characterizing the utility and limitations of repurposing an open-field optical imaging device for fluorescence-guided surgery in head and neck cancer patients. J Nucl Med 58:246–251
Tummers WS, Miller SE, Teraphongphom NT et al (2018) Intraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann Surg Oncol 25:1880–1888
Miller SE, Tummers WS, Teraphongphom N et al (2018) First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J Neurooncol 139:135–143
van Keulen S, Nishio N, Fakurnejad S et al (2019) The clinical application of fluorescence-guided surgery in head and neck cancer. J Nucl Med 60:758–763
Lu G, van den Berg NS, Martin BA et al (2020) Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: a phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol Hepatol 5:753–764
de Valk KS, Deken MM, Schaap DP et al (2021) Dose-finding study of a CEA-targeting agent, SGM-101, for intraoperative fluorescence imaging of colorectal cancer. Ann Surg Oncol 28:1832–1844
Tummers QRJG, Hoogstins CES, Gaarenstroom KN et al (2016) Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget 7:32144–32155
Tanyi JL, Randall LM, Chambers SK et al (2023) A phase III study of pafolacianine injection (OTL38) for intraoperative imaging of folate receptor-positive ovarian cancer (Study 006). J Clin Oncol 41:276–284
Butte PV, Mamelak A, Parrish-Novak J et al (2014) Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus 36:E1
Orosco RK, Tapia VJ, Califano JA et al (2018) Positive surgical margins in the 10 most common solid cancers. Sci Rep 8:5686
Williams AS, Hache KD (2014) Recognition and discrimination of tissue-marking dye color by surgical pathologists: recommendations to avoid errors in margin assessment. Am J Clin Pathol 142:355–361
Kiefer S, Huber J, Füllgraf H et al (2021) Alteration of tissue marking dyes depends on used chromogen during immunohistochemistry. Cells 10:835
van Keulen S, Nishio N, Birkeland A et al (2019) The sentinel margin: intraoperative ex vivo specimen mapping using relative fluorescence intensity. Clin Cancer Res 25:4656–4662
Kapoor S, Lu G, van den Berg NS et al (2021) Effect of formalin fixation for near-infrared fluorescence imaging with an antibody-dye conjugate in head and neck cancer patients. Mol Imaging Biol 23:270–276
Krishnan G, van den Berg NS, Nishio N et al (2022) Fluorescent molecular imaging can improve intraoperative sentinel margin detection in oral squamous cell carcinoma. J Nucl Med 63:1162–1168
Nishio N, van Keulen S, van den Berg NS et al (2020) Probe-based fluorescence dosimetry of an antibody-dye conjugate to identify head and neck cancer as a first step to fluorescence-guided tissue preselection for pathological assessment. Head Neck 42:59–66
Zhou Q, van den Berg NS, Kang W et al (2022) Factors for differential outcome across cancers in clinical molecule-targeted fluorescence imaging. J Nucl Med 63:1693–1700
Lu G, Fakurnejad S, Martin BA et al (2020) Predicting therapeutic antibody delivery into human head and neck cancers. Clin Cancer Res 26:2582–2594
Helman EE, Newman JR, Dean NR, Zhang W, Zinn KR, Rosenthal EL (2010) Optical imaging predicts tumor response to anti-EGFR therapy. Cancer Biol Ther 10:166–171
Heath CH, Deep NL, Sweeny L, Zinn KR, Rosenthal EL (2012) Use of panitumumab-IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol 19:3879–3887
Williams AS, Dakin Hache K (2014) Variable fidelity of tissue-marking dyes in surgical pathology. Histopathology 64:896–900
Acknowledgements
This work was supported partly by JSPS KAKENHI grant no. JP22K09723.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: TK, NN, JSP, JSL, ELR, and MEH; acquisition, analysis, or interpretation of data: all authors; drafting the work or revising it: TK, NN, JSP, JSL, and MEH; final approval of the version to be published: all authors; agreement to be accountable for all aspects: all authors.
Corresponding author
Ethics declarations
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Conflicts of Interest
The authors have no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11307_2023_1882_MOESM1_ESM.tif
Supplementary file1 Supplementary Figure. 1 Brightfield images of tissue-mimicking phantoms containing 6, 4, and 2 ng/mg concentrations of Panitumumab-IRDye800CW after painting eight tissue-marking dyes (TMDs) and 5% acetic acid solution on the top area of the phantoms.Each figure shows the phantom after painting black TMD at a, red TMD at b, yellow TMD at c, orange TMD at d, green TMD at e, lime TMD at f, blue TMD at g, and violet TMD at h on the top area of the phantom. (TIF 478 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kondo, T., Nishio, N., Park, J.S. et al. Identification of Optimal Tissue-Marking Dye Color for Pathological Evaluation in Fluorescence Imaging Using IRDye800CW. Mol Imaging Biol 26, 162–172 (2024). https://doi.org/10.1007/s11307-023-01882-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01882-x