Abstract
Purpose
We demonstrated earlier in mouse models of pancreatic ductal adenocarcinoma (PDA) that Ktrans derived from dynamic contrast-enhanced (DCE) MRI detected microvascular effect induced by PEGPH20, a hyaluronidase which removes stromal hyaluronan, leading to reduced interstitial fluid pressure in the tumor (Clinical Cancer Res (2019) 25: 2314–2322). How the choice of pharmacokinetic (PK) model and arterial input function (AIF) may impact DCE-derived markers for detecting such an effect is not known.
Procedures
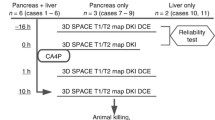
Retrospective analyses of the DCE-MRI of the orthotopic PDA model are performed to examine the impact of individual versus group AIF combined with Tofts model (TM), extended-Tofts model (ETM), or shutter-speed model (SSM) on the ability to detect the microvascular changes induced by PEGPH20 treatment.
Results
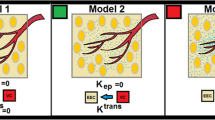
Individual AIF exhibit a marked difference in peak gadolinium concentration. However, across all three PK models, kep values show a significant correlation between individual versus group-AIF (p < 0.01). Regardless individual or group AIF, when kep is obtained from fitting the DCE-MRI data using the SSM, kep shows a significant increase after PEGPH20 treatment (p < 0.05 compared to the baseline); %change of kep from baseline to post-treatment is also significantly different between PEGPH20 versus vehicle group (p < 0.05). In comparison, when kep is derived from the TM, only the use of individual AIF leads to a significant increase of kep after PEGPH20 treatment, whereas the %change of kep is not different between PEGPH20 versus vehicle group. Group AIF but not individual AIF allows detection of a significant increase of Vp (derived from the ETM) in PEGPH20 versus vehicle group (p < 0.05). Increase of Vp is consistent with a large increase of mean capillary lumen area estimated from immunostaining.
Conclusion
Our results suggest that kep derived from SSM and Vp from ETM, both using group AIF, are optimal for the detection of microvascular changes induced by stroma-directed drug PEGPH20. These analyses provide insights in the choice of PK model and AIF for optimal DCE protocol design in mouse pancreatic cancer models.





Similar content being viewed by others
References
Hermann P, Kotek J, Kubíček V, Lukeš I (2008) Gadolinium(III) complexes as MRI contrast agents: ligand design and properties of the complexes. Dalton Trans 23:3027–3047. https://doi.org/10.1039/B719704G
Park JJ, Kim CK, Park SY et al (2014) Assessment of early response to concurrent chemoradiotherapy in cervical cancer: value of diffusion-weighted and dynamic contrast-enhanced MR imaging. Magn Reson Imaging 32:993–1000. https://doi.org/10.1016/j.mri.2014.05.009
Kang SR, Kim HW, Kim HS (2020) Evaluating the relationship between dynamic contrast-enhanced MRI (DCE-MRI) parameters and pathological characteristics in breast cancer. J Magn Reson Imaging 52:1360–1373. https://doi.org/10.1002/jmri.27241
Cheng Q, Huang J, Liang J et al (2020) The diagnostic performance of DCE-MRI in evaluating the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Front Oncol 10:93. https://doi.org/10.3389/fonc.2020.00093
O’Connor JPB, Jackson A, Parker GJM et al (2012) Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9:167–177. https://doi.org/10.1038/nrclinonc.2012.2
Duan C, Kallehauge JF, Bretthorst GL et al (2017) Are complex DCE-MRI models supported by clinical data? Magn Reson Med 77:1329–1339. https://doi.org/10.1002/mrm.26189
Inglese M, Ordidge KL, Honeyfield L et al (2019) Reliability of dynamic contrast-enhanced magnetic resonance imaging data in primary brain tumours: a comparison of Tofts and shutter speed models. Neuroradiology 61:1375–1386. https://doi.org/10.1007/s00234-019-02265-2
Khalifa F, Soliman A, El-Baz A et al (2014) Models and methods for analyzing DCE-MRI: a review. Med Phys 41:124301. https://doi.org/10.1118/1.4898202
Huang W, Chen Y, Fedorov A et al (2016) The impact of arterial input function determination variations on prostate dynamic contrast-enhanced magnetic resonance imaging pharmacokinetic modeling: a multicenter data analysis challenge. Tomography (Ann Arbor, Mich) 2:56–66. https://doi.org/10.18383/j.tom.2015.00184
Yang C, Karczmar GS, Medved M, Stadler WM (2004) Estimating the arterial input function using two reference tissues in dynamic contrast-enhanced MRI studies: fundamental concepts and simulations. Magn Reson Med 52:1110–1117. https://doi.org/10.1002/mrm.20243
Pickup S, Zhou R, Glickson J (2003) MRI estimation of the arterial input function in mice. Acad Radiol 10:963–968. https://doi.org/10.1016/s1076-6332(03)00291-5
Li X, Welch EB, Arlinghaus LR et al (2011) A novel AIF tracking method and a comparison of DCE-MRI parameters using individual and population based AIFs in human breast cancer. Phys Med Biol 56:5753–5769. https://doi.org/10.1088/0031-9155/56/17/018
Fluckiger JU, Schabel MC, DiBella EVR (2010) Toward local arterial input functions in dynamic contrast-enhanced MRI. J Magn Reson Imaging 32:924–934. https://doi.org/10.1002/jmri.22339
(2012) QIBA-RSNA, QIBA profile: DCE-MRI quantification (DCEMRI-Q),. https://qibawiki.rsna.org/index.php/Profiles. https://qibawiki.rsna.org/images/1/1f/QIBA_DCE-MRI_Profile-Stage_1-Public_Comment.pdf. Accessed 12 Jan 2023
Cao J, Pickup S, Clendenin C et al (2019) Dynamic contrast-enhanced mri detects responses to stroma-directed therapy in mouse models of pancreatic ductal adenocarcinoma. Clin Cancer Res 25:2314–2322. https://doi.org/10.1158/1078-0432.CCR-18-2276
Jacobetz MA, Chan DS, Neesse A et al (2013) Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62:112–120. https://doi.org/10.1136/gutjnl-2012-302529
Provenzano PP, Cuevas C, Chang AE et al (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429. https://doi.org/10.1016/j.ccr.2012.01.007
Provenzano PP, Hingorani SR (2013) Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 108:1–8. https://doi.org/10.1038/bjc.2012.569
Thompson CB, Shepard HM, O’Connor PM et al (2010) Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 9:3052–3064. https://doi.org/10.1158/1535-7163.Mct-10-0470
Zhou R, Pickup S, Yankeelov TE et al (2004) Simultaneous measurement of arterial input function and tumor pharmacokinetics in mice by dynamic contrast enhanced imaging: effects of transcytolemmal water exchange. Magn Reson Med 52:248–257. https://doi.org/10.1002/mrm.20143
Loveless ME, Halliday J, Liess C et al (2012) A quantitative comparison of the influence of individual versus population-derived vascular input functions on dynamic contrast enhanced-MRI in small animals. Magn Reson Med 67:226–236. https://doi.org/10.1002/mrm.22988
Yankeelov TE, Luci JJ, Lepage M et al (2005) Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging 23:519–529. https://doi.org/10.1016/j.mri.2005.02.013
Yankeelov TE, Cron GO, Addison CL et al (2007) Comparison of a reference region model with direct measurement of an AIF in the analysis of DCE-MRI data. Magn Reson Med 57:353–361. https://doi.org/10.1002/mrm.21131
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging: JMRI 7:91–101. https://doi.org/10.1002/jmri.1880070113
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging: JMRI 10:223–232. https://doi.org/10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s
Yankeelov TE, Rooney WD, Li X, Springer CS (2003) Variation of the relaxographic “shutter-speed” for transcytolemmal water exchange affects the CR bolus-tracking curve shape. Magn Reson Med 50:1151–1169. https://doi.org/10.1002/mrm.10624
Li X, Rooney WD, Springer CS (2005) A unified magnetic resonance imaging pharmacokinetic theory: intravascular and extracellular contrast reagents. Magn Reson Med 54:1351–1359. https://doi.org/10.1002/mrm.20684
Huang W, Li X, Chen Y et al (2014) Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl Oncol 7:153–166. https://doi.org/10.1593/tlo.13838
Kim S, Quon H, Loevner LA et al (2007) Transcytolemmal water exchange in pharmacokinetic analysis of dynamic contrast-enhanced MRI data in squamous cell carcinoma of the head and neck. J Magn Reson Imaging 26:1607–1617. https://doi.org/10.1002/jmri.21207
Huang W, Li X, Morris EA et al (2008) The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumors in vivo. Proc Natl Acad Sci U S A 105:17943–17948. https://doi.org/10.1073/pnas.0711226105
Gillis A, Gray M, Burstein D (2002) Relaxivity and diffusion of gadolinium agents in cartilage. Magn Reson Med 48:1068–1071. https://doi.org/10.1002/mrm.10327
Donahue KM, Burstein D, Manning WJ, Gray ML (1994) Studies of Gd-DTPA relaxivity and proton exchange rates in tissue. Magn Reson Med 32:66–76. https://doi.org/10.1002/mrm.1910320110
Li X, Cai Y, Moloney B et al (2016) Relative sensitivities of DCE-MRI pharmacokinetic parameters to arterial input function (AIF) scaling. J Magn Reson 269:104–112. https://doi.org/10.1016/j.jmr.2016.05.018
Sherman MH, Yu RT, Engle DD et al (2014) Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159:80–93. https://doi.org/10.1016/j.cell.2014.08.007
Chauhan VP, Martin JD, Liu H et al (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4:2516. https://doi.org/10.1038/ncomms3516
Li X, Priest RA, Woodward WJ et al (2013) Feasibility of shutter-speed DCE-MRI for improved prostate cancer detection. Magn Reson Med 69:171–178. https://doi.org/10.1002/mrm.24211
Buckley DL (2019) Shutter-speed dynamic contrast-enhanced MRI: Is it fit for purpose? Magn Reson Med 81:976–988. https://doi.org/10.1002/mrm.27456
Bai R, Wang B, Jia Y et al (2020) Shutter-speed DCE-MRI analyses of human glioblastoma multiforme (GBM) data. J Magn Reson Imaging 52:850–863. https://doi.org/10.1002/jmri.27118
Chawla S, Loevner LA, Kim SG et al (2018) Dynamic contrast-enhanced MRI–derived intracellular water lifetime (τi): a prognostic marker for patients with head and neck squamous cell carcinomas. AJNR Am J Neuroradiol 39:138–144. https://doi.org/10.3174/ajnr.A5440
Springer CS (2018) Using 1H2O MR to measure and map sodium pump activity in vivo. J Magn Reson 291:110–126. https://doi.org/10.1016/j.jmr.2018.02.018
Springer CS, Li X, Tudorica LA et al (2014) Intratumor mapping of intracellular water lifetime: metabolic images of breast cancer? NMR in Biomedicine 27:760–773. https://doi.org/10.1002/nbm.3111
Romanello Joaquim M, Furth EE, Fan Y et al (2022) DWI metrics differentiating benign intraductal papillary mucinous neoplasms from invasive pancreatic cancer: a study in GEM models. Cancers 14:4017. https://doi.org/10.3390/cancers14164017
Lin W, Guo J, Rosen MA, Song HK (2008) Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn Reson Med 60:1135–1146. https://doi.org/10.1002/mrm.21740
Heacock L, Gao Y, Heller SL et al (2017) Comparison of conventional DCE-MRI and a novel golden-angle radial multicoil compressed sensing method for the evaluation of breast lesion conspicuity. J Magn Reson Imaging 45:1746–1752. https://doi.org/10.1002/jmri.25530
Pickup S, Romanello M, Gupta M et al (2022) Dynamic contrast-enhanced MRI in the abdomen of mice with high temporal and spatial resolution using stack-of-stars sampling and KWIC reconstruction. Tomography 8:2113–2128. https://doi.org/10.3390/tomography8050178
Cárdenas-Rodríguez J, Howison CM, Pagel MD (2013) A linear algorithm of the reference region model for DCE-MRI is robust and relaxes requirements for temporal resolution. Magn Reson Imaging 31:497–507. https://doi.org/10.1016/j.mri.2012.10.008
Paudyal R, Poptani H, Cai K et al (2013) Impact of transvascular and cellular-interstitial water exchange on dynamic contrast-enhanced magnetic resonance imaging estimates of blood to tissue transfer constant and blood plasma volume. J Magn Reson Imaging 37:435–444. https://doi.org/10.1002/jmri.23837
Zhang J, Kim S (2014) Uncertainty in MR tracer kinetic parameters and water exchange rates estimated from T1-weighted dynamic contrast enhanced MRI. Magn Reson Med 72:534–545. https://doi.org/10.1002/mrm.24927
Acknowledgements
The authors thank the support from the Small Animal Imaging Facility (SAIF) of the Department of Radiology at the University of Pennsylvania.
Data Availability Statement
Image data will be shared at a data repository built for Penn Quantitative Imaging Resource for Pancreatic Cancer (https://pennpancreaticcancerimagingresource. github.io/data.html/).
Funding
This project is supported by NIH U24CA231858 (Penn Quantitative Imaging Resource for Pancreatic Cancer) and P30-CA-016520-42(Abramson Cancer Center).
Author information
Authors and Affiliations
Contributions
Conceptualization, RZ, J.C.; methodology development, J.C., S.P., and R.Z.; software, S.P.; data analysis, J.C., S.P., and R.Z.; data curation, J.C., S.P.; writing—original draft preparation RZ and J.C; writing review and editing, RZ, JC, MR, and S.P.; visualization, J.C., S.P., supervision, R.Z.; funding acquisition, R.Z. and MR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of the University of Pennsylvania (Protocol # 806083 approved on 03/12/2017).
Informed Consent Statement
Not applicable (no human study involved).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, J., Pickup, S., Rosen, M. et al. Impact of Arterial Input Function and Pharmacokinetic Models on DCE-MRI Biomarkers for Detection of Vascular Effect Induced by Stroma-Directed Drug in an Orthotopic Mouse Model of Pancreatic Cancer. Mol Imaging Biol 25, 638–647 (2023). https://doi.org/10.1007/s11307-023-01824-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01824-7