Abstract
Background
Intraoperative molecular imaging (IMI)-guided resections have been shown to improve oncologic outcomes for patients undergoing surgery for solid malignancies. The technology utilizes fluorescent tracers targeting cancer cells without the use of any ionizing radiation. However, currently available targeted IMI tracers are effective only for tumors with a highly specific receptor expression profile, and there is an unmet need for IMI tracers to label a broader range of tumor types. Here, we describe the development and testing of a novel tracer (CR)-S0456) targeted to the sodium multivitamin transporter (SMVT).
Methods
Preclinical models of fibrosarcoma (HT-1080), lung (A549), breast (4T1), and renal cancers (HEK-293 T) in vitro and in vivo were used for assessment of (CR)-S0456 specific tumor labeling via sodium-mediated SMVT uptake in dipotassium phosphate or choline chloride-containing media buffer. Additionally, pharmacologic inhibition of multiple intracellular coenzyme-R obligate signaling pathways, including holocarboxylase synthetase (sulconazole nitrate), PI3K/AKT/mTOR (omipalisib), and calmodulin-dependent phosphatase (calmidazolium), were investigated to assess (CR)-S0456 uptake kinetics. Human fibrosarcoma-bearing xenografts in athymic nude mice were used for tumor and metabolic-specific labeling. Novel NIR needle confocal laser endomicroscopic (nCLE) intratumoral sampling was performed to demonstrate single-cell specific labeling by CR-S0456.
Results
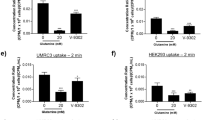
CR-S0456 localization in vitro correlated with highly proliferative cell lines (MTT) and doubling time (p < 0.05) with the highest microscopic fluorescence detected in aggressive human fibrosarcomas (HT-1080). Coenzyme-R-specific localization was demonstrated to be SMVT-specific after competitive inhibition of internal localization with excess administration of pantothenic acid. Inhibiting the activity of SMVT by affecting sodium ion hemostasis prevented the complete uptake of CR-S0456. In vivo validation demonstrated (CR)-S0456 localization to xenograft models with accurate identification of primary tumors as well as margin assessment down to 1 mm3 tumor volume. Systemic treatment of xenograft-bearing mice with a dual PI3K/mTOR inhibitor suppressed intratumoral cell signaling and (CR)-S0456 uptake via a reduction in SMVT expression. Novel analysis of in vivo intratumoral cytologic fluorescence using near-infrared confocal laser endomicroscopy demonstrated the absence of coenzyme-R-mediated NIR fluorescence but not fibroblast activation protein (FAP)-conjugated fluorochrome, indicating specific intracellular inhibition of coenzyme-R obligate pathways.
Conclusion
These findings suggest that a SMVT-targeted NIR contrast agent can be a suitable tracer for imaging a wide range of malignancies as well as evaluating metabolic response to systemic therapies, similar to PET imaging with immune checkpoint inhibitors.








Similar content being viewed by others
Data Availability
The data generated in this study are displayed in the manuscript and supplemental files. Data is available upon request from the corresponding author.
Material Availability
The data generated in this study are displayed in the manuscript and supplemental files. Data is available upon request from the corresponding author.
Abbreviations
- IMI:
-
Intraoperative molecular imaging
- CR:
-
Coenzyme R
- SMVT:
-
Sodium dependent multivitamin transporter
- NIR:
-
Near infrared
- HLCS:
-
Holocarboxylase synthetase
References
Fedor D, Johnson WR, Singhal S (2013) Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol 22:156–161
Azari F et al (2022) State of the art: precision surgery guided by intraoperative molecular imaging. J Nucl Med. https://doi.org/10.2967/jnumed.121.263409
Chi C et al (2014) Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 4:1072–1084
Azari F et al (2021) Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. JBO 26:050901
Sukjoi W et al (2020) Overexpression of holocarboxylase synthetase predicts lymph node metastasis and unfavorable prognosis in breast cancer. Anticancer Res 40:4557–4565
Scheerger SB, Zempleni J (2003) Expression of oncogenes depends on biotin in human small cell lung cancer cells NCI-H69. Int J Vitam Nutr Res 73:461–467
Kiesel VA et al (2021) Pyruvate carboxylase and cancer progression. Cancer Metab 9:20
Zempleni J, Liu D, Camara DT, Cordonier EL (2014) Novel roles of holocarboxylase synthetase in gene regulation and intermediary metabolism. Nutr Rev 72:369–376
Solórzano-Vargas RS, Pacheco-Alvarez D, León-Del-Río A (2002) Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. Proc Natl Acad Sci 99:5325–5330
Yoon J et al (2021) A chemical biology approach reveals a dependency of glioblastoma on biotin distribution. Sci Adv 7:eabf6033
Vadlapudi AD, Vadlapatla RK, Mitra AK (2012) Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr Drug Targets 13:994–1003
Patel M, Vadlapatla RK, Shah S, Mitra AK (2012) Molecular expression and functional activity of sodium dependent multivitamin transporter in human prostate cancer cells. Int J Pharm 436:324–331
Fam KT, Collot M, Klymchenko AS (2020) Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chem Sci 11:8240–8248
Cerami E et al (2012) The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404
Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the surgical benefit of utilizing 5-ALA for fluorescence-guided surgery of malignant gliomas? Neurosurg 77:663–673
Lee SJ, Choi YY, Kim C, Chung MS (2017) Correlations between tumor to background ratio on breast-specific gamma imaging and prognostic factors in breast cancer. J Korean Med Sci 32:1031–1037
Lee JY-K et al (2016) Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurg 79:856–871
Azari F, Kennedy G, Singhal S (2020) Intraoperative detection and assessment of lung nodules. Surg Oncol Clin 29:525–541
Pinon V, Ravanel S, Douce R, Alban C (2005) Biotin synthesis in plants. The first committed step of the pathway is catalyzed by a cytosolic 7-keto-8-aminopelargonic acid synthase. Plant Physiol 139:1666–1676
Song X et al (2021) Construction of a biotin-targeting drug delivery system and its near-infrared theranostic fluorescent probe for real-time image-guided therapy of lung cancer. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2021.08.111
Sun T et al (2020) Integrated profiling identifies SLC5A6 and MFAP2 as novel diagnostic and prognostic biomarkers in gastric cancer patients. Int J Oncol 56:460–469
Hassan YI, Zempleni J (2006) Epigenetic regulation of chromatin structure and gene function by biotin. J Nutr 136:1763–1765
Park S, Kim E, Kim WY, Kang C, Kim JS (2015) Biotin-guided anticancer drug delivery with acidity-triggered drug release. Chem Commun 51:9343–9345
Vadlapudi AD, Vadlapatla RK, Pal D, Mitra AK (2013) Biotin uptake by T47D breast cancer cells: functional and molecular evidence of sodium-dependent multivitamin transporter (SMVT). Int J Pharm 441:535–543
Said HM et al (1998) Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol Cell Physiol 275:C1365–C1371
Stanley JS, Griffin JB, Zempleni J (2001) Biotinylation of histones in human cells. Eur J Biochem 268:5424–5429
Treasure T, Fiorentino F, Scarci M, Møller H, Utley M (2012) Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2(5):e001736. https://doi.org/10.1136/bmjopen-2012-001736
Digesu CS, Wiesel O, Vaporciyan AA, Colson YL (2016) Management of sarcoma metastases to the lung. Surg Oncol Clin N Am 25:721–733
Acknowledgements
We would like to thank Drs. Steven Albelda and Philip Low for material and cell line support.
Funding
Dr. Azari was supported by the training grant in surgical oncology from the National Institutes of Health (T32CA251063-01), the Society of Thoracic Surgeons Thoracic Surgery Foundation Research Award, and the Stephen C. Cheung Award in Surgical Oncology. Dr. Singhal was supported by the National Institutes of Health (R01 CA193556) and the State of Pennsylvania Health Research Formula Fund. Dr. Kennedy was supported by the American Philosophical Society and the National Institutes of Health (F32 CA254210-01). Dr. Sullivan was supported by NIH T32 postdoctoral fellowship (NIH T32 CA009140).
Author information
Authors and Affiliations
Contributions
FA, GTK, JD, and SS: study design, data validation, manuscript draft, manuscript finalization, and review. AC, NTS, AS, EB, BA, and EE: data acquisition and experiment conduction.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All experiments have been approved by the University of Pennsylvania Institutional Review Board. Animal studies were performed according to protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health guidelines.
Consent for Publication
N/A
Competing Interests
S.S. has been named a co-inventor and submitted a patent to the USPTO regarding the fluorochrome. In order to protect intellectual property while the patent application process is pending, the structure is omitted from the manuscript. The structure is readily available by contacting the corresponding author and the University of Pennsylvania Intellectual Protection Office.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 4795 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azari, F., Kennedy, G.T., Chang, A. et al. Sodium Multivitamin Transporter-Targeted Fluorochrome Facilitates Enhanced Metabolic Evaluation of Tumors Through Coenzyme-R Dependent Intracellular Signaling Pathways. Mol Imaging Biol 25, 569–585 (2023). https://doi.org/10.1007/s11307-022-01792-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01792-4