Abstract
Purpose
Objectives of the study are to analyze the correlation between [68Ga]DOTATATE positron emission tomography (PET)/X-ray computed tomography (CT) measurements and various biological characteristics of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs), and to determine optimal cutoff value of SUVmax (standard uptake value) to differentiate neuroendocrine tumors (NETs) and neuroendocrine cancers (NECs).
Procedures
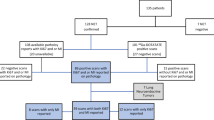
Of the GEP-NEN cases (73 males, 53 females; age 18–77 years) with pathologically proven primary and/or metastatic lesions, 126 were studied. All of the short axes of lesions were larger than 0.5 cm in order to avoid the partial volume effect. Patients fasted for 6 h before the PET/CT scans. The dose of [68Ga]DOTATATE was 100–200 MBq and the acquisition began at 1 h after injection. The lesion with the highest SUVmax in each patient was analyzed.
Results
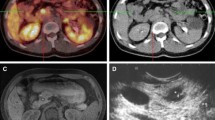
In the total sample, the sensitivity of [68Ga]DOTATATE was 69.05 %. The sensitivities were significantly different among G1, G2, and G3 groups (72.22 %, 91.53 %, and 40.82 %, respectively; p < 0.01). The SUVmax of the G3 group was lowest. We also found that the sensitivity and SUVmax were significantly higher (p < 0.05) in patients with pancreatic NENs (Pan-NENs) than in patients with gastrointestinal NENs (Gi-NENs) and unknown primary NENs (Up-NENs). A significant negative correlation between SUVmax and Ki-67 was found (r = − 0.429, p < 0.01). Using SUVmax to differentiate neuroendocrine tumors (NETs) and neuroendocrine cancers (NECs), the area under the ROC curve (AUC) was 0.771 and the cutoff value of SUVmax was 11.25 (sensitivity 79.2 %, specificity 65.3 %). However, Pan-NENs did not show any statistical significance results in correlation and ROC analysis.
Conclusion
[68Ga]DOTATATE PET/CT results showed a negative correlation with GEP-NEN cell proliferation and were complementary to Ki-67. Pan-NENs were different from Gi-NENs and Up-NENs when compared to somatostatin receptor expression.





Similar content being viewed by others
References
Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Diaz-Perez JA, Martinez del Prado MP, Alonso Orduna V, Sevilla-Garcia I, Villabona-Artero C, Beguiristain-Gomez A, Llanos-Munoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jimenez-Fonseca P, Teule A, Sastre-Valera J, Benavent-Vinuelas M, Monleon A, Salazar R (2010) Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol 21:1794–1803
Rindi G, Petrone G, Inzani F (2014) The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol 25:186–192
Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B (2007) TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 451:757–762
Yang Z, Tang LH, Klimstra DS (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–860
Adesoye T, Daleo MA, Loeffler AG et al (2015) Discordance of histologic grade between primary and metastatic neuroendocrine carcinomas. Ann Surg Oncol Suppl 3:S817–S821
Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F (2008) Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 26:4311–4318
Singh S, Hallet J, Rowsell C, Law CH (2014) Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur J Surg Oncol 40:1517–1522
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AMM, Santini D, Ceccarelli C, Nori F, Franchi R, Corinaldesi R, Tomassetti P (2010) Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med 51:353–359
Tirosh A, Papadakis GZ, Millo C et al (2018) Prognostic utility of Total 68Ga-DOTATATE-avid tumor volume in patients with neuroendocrine tumors. Gastroenterology 154:998–1008
Reubi JC, Kvols L, Krenning E, Lamberts SW (1991) In vitro and in vivo detection of somatostatin receptors in human malignant tissues. Acta Oncol 30:463–468
Kvols LK, Reubi JC, Horisberger U et al (1992) The presence of somatostatin receptors in malignant neuroendocrine tumor tissue predicts responsiveness to octreotide. Yale J Biol Med 65:505–518
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Gerdes J, Lemke H, Baisch H et al (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322
Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, Lafitte JJ, Sculier JP (2004) Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 91:2018–2025
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139:539–552
Klimstra DS (2013) Pathology reporting of neuroendocrine tumors: essential elements for accurate diagnosis, classification, and staging. Semin Oncol 40:23–36
Dhall D, Mertens R, Bresee C, Parakh R, Wang HL, Li M, Dhall G, Colquhoun SD, Ines D, Chung F, Yu R, Nissen NN, Wolin E (2012) Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum Pathol 43:489–495
Miller HC, Drymousis P, Flora R, Goldin R, Spalding D, Frilling A (2014) Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J Surg 38:1353–1361
O’Toole D, Saveanu A, Couvelard A, Gunz G, Enjalbert A, Jaquet P, Ruszniewski P, Barlier A (2006) The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol 155:849–857
Acknowledgments
We acknowledge the staff of the departments involved in the study, the referring clinicians for their input into management data, and the patients who participated in the study. We particularly thank Judy Buchanan, Johns Hopkins University School of Medicine, USA, for her suggestions of the manuscript.
Funding
This study was funded by Science Foundation of Peking University Cancer Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Li, N., Li, J. et al. The Correlation Between [68Ga]DOTATATE PET/CT and Cell Proliferation in Patients With GEP-NENs. Mol Imaging Biol 21, 984–990 (2019). https://doi.org/10.1007/s11307-019-01328-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01328-3