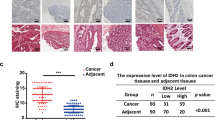
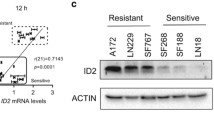
Abstract
Purpose
Although glucose transporter 1 (GLUT1) and hexokinase 2 (HK2) are known as major proteins involved in the molecular mechanisms for accumulating 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) in cancer cells, sometimes, [18F] FDG accumulation cannot be explained by the expression of these two proteins. We investigated the involvement of adenine nucleotide translocase 2 (ANT2), which catalyzes ADP/ATP exchange at the mitochondrial inner membrane, in [18F] FDG accumulation.
Procedures
ANT2 expression was evaluated in various cancer cell lines and human cancer tissues (microarrays) using western blot and immunohistochemical (IHC) staining, respectively. The expression levels of ANT2 were compared to [18F] FDG accumulation and pathologic findings, including differentiation grade. Additionally, we modulated ANT2 expression levels using ANT2 siRNA and an ANT2 expression vector in cancer cells and murine xenografted tumors.
Results
[18F] FDG accumulation correlated with ANT2 expression in various cancer cell lines; this was not explained by GLUT1 and/or HK2 expression. At both the cell and tissue levels, ANT2 expression was high in less-differentiated or more malignant type of cancers. [18F] FDG accumulation changed according to the modulation of the ANT2 expression level.
Conclusion
In various cancer cells and tissues, the expression levels of ANT2 explained [18F] FDG accumulation better than those of GLUT1 and HK2. ANT2 can be used as a marker of dedifferentiated pathology and aggressiveness of cancer.






Similar content being viewed by others
References
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13:472–482
Kubota K, Matsuzawa T, Fujiwara T, Ito M, Hatazawa J, Ishiwata K, Iwata R, Ido T (1990) Differential diagnosis of lung tumor with positron emission tomography: a prospective study. J Nucl Med 31:1927–1932
Gambhir SS, Czernin J, Schwimmer J et al (2001) A tabulated summary of the FDG PET literature. J Nucl Med 42(Suppl 5):1S–93S
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Pieterman RM, van Putten JW, Meuzelaar JJ et al (2000) Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 343:254–261
Antoniou AJ, Marcus C, Tahari AK, Wahl RL, Subramaniam RM (2014) Follow-up or surveillance 18F-FDG PET/CT and survival outcome in lung cancer patients. J Nucl Med 55:1062–1068
Smith TA (1998) FDG uptake, tumour characteristics and response to therapy: a review. Nucl Med Commun 19:97–105
Pauwels EK, McCready VR, Stoot JH et al (1998) The mechanism of accumulation of tumor-localising radiopharmaceuticals. Eur J Nucl Med 25:277–305
Gallagher BM, Fowler JS, Gutterson NI, MacGregor R, Wan CN, Wolf AP (1978) Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med 19:1154–1161
Pauwels EK, Ribeiro MJ, Stoot JH et al (1998) FDG accumulation and tumor biology. Nucl Med Biol 25:317–322
Szablewski L (2013) Expression of glucose transporters in cancers. Biochim Biophys Acta 1835:164–169
Smith TA (2000) Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci 57:170–178
Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16:635–649
Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, Jha AK, Smolen GA, Clasquin MF, Robey RB, Hay N (2013) Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24:213–228
Smith TA (2001) The rate-limiting step for tumor [18F]fluoro-2-deoxy-D-glucose (FDG) incorporation. Nucl Med Biol 28:1–4
Mertens K, Mees G, Lambert B, van de Wiele C, Goethals I (2012) In vitro 2-deoxy-2-[18F]fluoro-D-glucose uptake: practical considerations. Cancer Biother Radiopharm 27:183–188
Jadvar H, Alavi A, Gambhir SS (2009) 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med 50:1820–1827
Alvarez JV, Belka GK, Pan TC, Chen CC, Blankemeyer E, Alavi A, Karp JS, Chodosh LA (2014) Oncogene pathway activation in mammary tumors dictates FDG-PET uptake. Cancer Res 74:7583–7598
Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, Hricak H, Klimstra D, Fong Y (2007) 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med 48:771–775
Robey RB, Hay N (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696
Golshani-Hebroni SG, Bessman SP (1997) Hexokinase binding to mitochondria: a basis for proliferative energy metabolism. J Bioenerg Biomembr 29:331–338
Pedersen PL (2008) Voltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. J Bioenerg Biomembr 40:123–126
Gogvadze V, Zhivotovsky B, Orrenius S (2010) The Warburg effect and mitochondrial stability in cancer cells. Mol Asp Med 31:60–74
Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25:4777–4786
Klingenberg M (2008) The ADP and ATP transport in mitochondria and its carrier. Biochim Biophys Acta 1778:1978–2021
Le Bras M, Borgne-Sanchez A, Touat Z et al (2006) Chemosensitization by knockdown of adenine nucleotide translocase-2. Cancer Res 66:9143–9152
Chevrollier A, Loiseau D, Reynier P, Stepien G (2011) Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim Biophys Acta 1807:562–567
Gavaldà-Navarro A, Mampel T, Viñas O (2016) Changes in the expression of the human adenine nucleotide translocase isoforms condition cellular metabolic/proliferative status. Open Biol 6:150108
Perisic L, Hedin E, Razuvaev A, Lengquist M, Osterholm C, Folkersen L, Gillgren P, Paulsson-Berne G, Ponten F, Odeberg J, Hedin U (2013) Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol 33:2432–2443
Chiu YL, Rana TM (2003) siRNA function in RNAi: a chemical modification analysis. RNA 9:1034–1048
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy. Semin Cancer Biol 19:17–24
Stepien G, Torroni A, Chung AB et al (1992) Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem 267:14592–14597
Brenner C, Subramaniam K, Pertuiset C, Pervaiz S (2011) Adenine nucleotide translocase family: four isoforms for apoptosis modulation in cancer. Oncogene 30:883–895
Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N (2007) Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem 282:29658–29666
Chevrollier A, Loiseau D, Chabi B, Renier G, Douay O, Malthièry Y, Stepien G (2005) ANT2 isoform required for cancer cell glycolysis. J Bioenerg Biomembr 37:307–316
Kim HS, Je JH, Son TG, Park HR, Ji ST, Pokharel YR, Jeon HM, Kang KW, Kang HS, Chang SC, Kim HS, Chung HY, Lee J (2012) The hepatoprotective effects of adenine nucleotide translocator-2 against aging and oxidative stress. Free Radic Res 46:21–29
Vesselle H, Schmidt RA, Pugsley JM et al (2000) Lung cancer proliferation correlates with [F-18] fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 6:3837–3844
Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, Vallières E, Wood DE (2008) Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol 3:971–978
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, Iwaisako K, Ikai I, Uemoto S (2007) Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res 13(2 Pt 1):427–433
Abraham T, Schöder H (2011) Thyroid cancer--indications and opportunities for positron emission tomography/computed tomography imaging. Semin Nucl Med 41:121–138
Bogsrud TV, Karantanis D, Nathan MA, Mullan BP, Wiseman GA, Kasperbauer JL, Reading CC, Hay ID, Lowe VJ (2008) 18F-FDG PET in the management of patients with anaplastic thyroid carcinoma. Thyroid 18:713–719
Are C, Shaha AR (2006) Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 13:453–464
Jang JY, Kim YG, Nam SJ, Keam B, Kim TM, Jeon YK, Kim CW (2016) Targeting adenine nucleotide translocase-2 (ANT2) to overcome resistance to epidermal growth factor receptor tyrosine kinase inhibitor in non-small cell lung Cancer. Mol Cancer Ther 15:1387–1396
Prabhu D, Goldstein AC, El-Khoury R et al (2015) ANT2-defective fibroblasts exhibit normal mitochondrial bioenergetics. Mol Genet Metab Rep 3:43–46
Cho J, Zhang Y, Park SY, Joseph AM, Han C, Park HJ, Kalavalapalli S, Chun SK, Morgan D, Kim JS, Someya S, Mathews CE, Lee YJ, Wohlgemuth SE, Sunny NE, Lee HY, Choi CS, Shiratsuchi T, Oh SP, Terada N (2017) Mitochondrial ATP transporter depletion protects mice against liver steatosis and insulin resistance. Nat Commun 8:14477
Funding
This work was supported by grants from the National Research Foundation, Ministry of Science and ICT (2011-0030001 for J-K.C. and H.Y., 2017-R1A2B4012813 for H.Y.), and the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1072 for K.W.K, G.J.C. and H.Y., HI15C2971 for H.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study has been approved by the Institutional Review Board and all subjects signed an informed consent form.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PDF 130 kb)
Rights and permissions
About this article
Cite this article
Lee, CH., Kim, M.J., Lee, H.H. et al. Adenine Nucleotide Translocase 2 as an Enzyme Related to [18F] FDG Accumulation in Various Cancers. Mol Imaging Biol 21, 722–730 (2019). https://doi.org/10.1007/s11307-018-1268-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1268-x