Abstract
Background
Due to the limited availability of suitable positron emission tomography (PET) tracers, the majority of myocardial perfusion imaging (MPI) scans is performed using SPECT rather than PET.
Aim
The aim of this study is to design and synthesize carbon-11-labeled ammonium salt derivatives and explore their structure–activity relationship (SAR) and their potential as PET–MPI agents.
Methods and Results
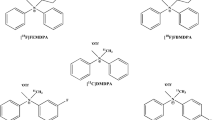
Three carbon-11-labeled ammonium salts were developed. SAR of the labeled compounds were explored vis-à-vis the effects of charge density and lipophilicity on the distribution kinetics in mice. These studies pointed at [11C]4 as the lead compound. Comparative microPET/CT scans in healthy rats, using both [11C]4 and [13 N]–NH3, substantiated the potential of [11C]4 ([11C]-DMDPA). A proof of concept for the potential of radiolabeled ammonium salts as MPI agents has been demonstrated in a newly developed swine model of permanent partial coronary artery occlusion.
Conclusions
SAR studies of 11C-labeled ammonium salts suggest that both lipophilicity and charge density affect the performance of these compounds as MPI probes. In a swine model, the labeled lead successfully visualized the defect regions in the myocardium. The data presented call for the development of fluorine-18 analogues, to increase clinical impact.







Similar content being viewed by others
References
Marcassa C, Bax JJ, Bengel F, Hesse B, Petersen CL, Reyes E et al (2008) Clinical value, cost-effectiveness, and safety of myocardial perfusion scintigraphy: a position statement. Eur Heart J 29:557–563
Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC (2007) Clinical myocardial perfusion PET/CT. J Nucl Med 48:783–793
Ilovich O, Billauer H, Dotan S, Freedman NM, Bocher M, Mishani E (2011) Novel and simple carbon-11-labeled ammonium salts as PET agents for myocardial perfusion imaging. Mol Imaging Biol 13:128–139
Nekolla SG, Saraste A (2011) Novel F-18-labeled PET myocardial perfusion tracers: bench to bedside. Curr Cardiol Rep 13:145–150
Di Carli MF, Hachamovitch R (2006) Should PET replace SPECT for evaluating CAD? The end of the beginning. J Nucl Cardiol 13:2–7
Glover DK, Gropler RJ (2007) Journey to find the ideal PET flow tracer for clinical use: are we there yet? J Nucl Cardiol 14:765–768
Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ et al (2008) Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med 49:630–636
Madar I, Ravert H, Nelkin B, Abro M, Pomper M, Dannals R et al (2007) Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging 34:2057–2065
Madar I, Ravert HT, Du Y, Hilton J, Volokh L, Dannals RF et al (2006) Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med 47:1359–1366
Marshall RC, Powers-Risius P, Reutter BW, O’Neil JP, La Belle M, Huesman RH et al (2004) Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial flow tracer: comparison to 201Tl. J Nucl Med 45:1950–1959
Yu M, Guaraldi M, Kagan M, Mistry M, McDonald J, Bozek J et al (2009) Assessment of 18F-labeled mitochondrial complex I inhibitors as PET myocardial perfusion imaging agents in rats, rabbits, and primates. Eur J Nucl Med Mol Imaging 36:63–72
Yu M, Nekolla SG, Schwaiger M, Robinson SP (2011) The next generation of cardiac positron emission tomography imaging agents: discovery of flurpiridaz F-18 for detection of coronary disease. Semin Nucl Med 41:305–313
Crouzel C, Långström B, Pike VW, Coenen HH (1987) Recommendations for a practical production of [11C]methyl iodide. Int J Radiat Appl Instrum Appl Radiat Isot 38:601–603
Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A et al (2009) Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation 119:2333–2342
Mock BH, Mulholland GK, Vavrek MT (1999) Convenient gas phase bromination of [11C]methane and production of [11C]methyl triflate. Nucl Med Biol 26:467–471
Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M et al (2007) Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol 14:782–788
Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH (1998) Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 193(Pt 1):105–119
Hughes HC (1986) Swine in cardiovascular research. Lab Anim Sci 36:348–350
Sherif HM, Saraste A, Weidl E, Weber AW, Higuchi T, Reder S et al (2009) Evaluation of a novel (18)F-labeled positron-emission tomography perfusion tracer for the assessment of myocardial infarct size in rats. Circulation 2:77–84
Shoup TM, Elmaleh DR, Brownell AL, Zhu A, Guerrero JL, Fischman AJ (2011) Evaluation of (4-[(1)F]Fluorophenyl)triphenylphosphonium ion. A potential myocardial blood flow agent for PET. Mol Imaging Biol 13:511–517
Rauch B, Helus F, Grunze M, Braunwell E, Mall G, Hasselbach W et al (1985) Kinetics of 13N-ammonia uptake in myocardial single cells indicating potential limitations in its applicability as a marker of myocardial blood flow. Circulation 71:387–393
Acknowledgments
The authors wish to thank Rami Marciano, Sassi Cohen, Tomer Yamin, Daniel Wajnblum, Orit Jacobson-Weiss, Darya Tsvirkun, and Marina Orevi for their technical help and their useful advice.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Ohad Ilovich and Galith Abourbeh equally contributed to this article.
Rights and permissions
About this article
Cite this article
Ilovich, O., Abourbeh, G., Bocher, M. et al. Structure–Activity Relationship and Preclinical Evaluation of Carbon-11-Labeled Ammonium Salts as PET–Myocardial Perfusion Imaging Agents. Mol Imaging Biol 14, 625–636 (2012). https://doi.org/10.1007/s11307-011-0539-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0539-6