Abstract
Introduction
A large scale population exposure to ionizing radiation during intentional or unintentional nuclear accidents undoubtedly generates a complex scenario with partial-body as well as total-body irradiated victims. A high throughput technique based rapid assessment method is an urgent necessity for stratification of exposed subjects independent of whether exposure is uniform total-body or non-homogenous partial-body.
Objective
Here, we used Nuclear Magnetic Resonance (NMR) based metabolomics approach to compare and identify candidate metabolites differentially expressed in total and partially irradiated mice model.
Methods
C57BL/6 male mice (8–10 weeks) were irradiated total-body or locally to thoracic, hind limb or abdominal regions with 10 Gy of gamma radiation. Urine samples collected at 24 h post irradiation were examined using high resolution NMR spectroscopy and the datasets were analysed using multivariate analysis.
Results
Multivariate and metabolic pathway analysis in urine samples collected at 24 h post-radiation exhibited segregation of all irradiated groups from controls. Metabolites associated with energy metabolism, gut flora metabolism and taurine were common to partial and total-body irradiation, thus making them potential candidates for radiation exposure. Nevertheless, a distinct metabolic pattern was observed in partial-body exposed groups with maximum changes observed in the hind limb region indicating differential tissue associated radiation sensitivity. The organ-specific changes may provide an early warning regarding the physiological system at risk after radiation injury.
Conclusion
The study affirms potentiality of metabolite markers and comparative analysis could be an important piece of information for an integrated solution to a complex research question in terms of radiation biomarkers.





Similar content being viewed by others
References
Allison, G. (2018). Nuclear terrorism: Did we beat the odds or change them? PRISM, NDU press, 7(3). https://ndupress.ndu.edu/Journals/PRISM/.
Anderson, R. M. (2019). Cytogenetic biomarkers of radiation exposure. Clinical Oncology, 31(5), 311–318.
Berkers, C. R., Maddocks, O. D. K., Cheung, E. C., Mor, I., & Vousden, K. H. (2013). Metabolic regulation by p53 family members. Cell Metabolism, 18(5), 617–633.
Bleijlevens, B., Shivarattan, T., van den Boom, K. S., de Haan, A., van der Zwan, G., Simpson, P. J., et al. (2012). Changes in protein dynamics of the DNA repair dioxygenase AlkB upon binding of Fe2+ and 2-oxoglutarate. Biochemistry, 51(16), 3334–3341.
Chen, C., Brenner, D. J., & Brown, T. R. (2011). Identification of urinary biomarkers from X-irradiated mice using NMR spectroscopy. Radiation Research, 175(5), 622–630.
Christophersen, O. A. (2012). Radiation protection following nuclear power accidents: A survey of putative mechanisms involved in the radioprotective actions of taurine during and after radiation exposure. Microbial Ecology in Health and Disease, 23(1), 14787.
Coy, S. L., Cheema, A. K., Tyburski, J. B., Laiakis, E. C., Collins, S. P., & Fornace, A. (2011). Radiation metabolomics and its potential in biodosimetry. International Journal of Radiation Biology, 87(8), 802–823.
Datta, K., Suman, S., Kallakury, B. V., & Fornace, A. J., Jr. (2012). Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE, 7(8), e42224.
Dilley, J. V. (1972). The origin of urinary taurine excretion during chronic radiation injury. Radiation Research, 50(1), 191–196.
Emwas, A. H., Roy, R., McKay, R. T., Tenori, L., Saccenti, E., Gowda, G. A. N., et al. (2019). NMR spectroscopy for metabolomics research. Metabolites, 9(7), 123.
Fazzino, F., Obregón, F., & Lima, L. (2010). Taurine and proliferation of lymphocytes in physically restrained rats. Journal of Biomedical Science, 17(Suppl 1), S24.
Fazzino, F., Urbina, M., Mata, S., & Lima, L. (2006). Taurine transport and transporter localization in peripheral blood lymphocytes of controls and major depression patients. Advances in Experimental Medicine and Biology, 583, 423–426.
Gan, W. Z., Ramachandran, V., Lim, C. S. Y., & Koh, R. Y. (2019). Omics-based biomarkers in the diagnosis of diabetes. Journal of Basic and Clinical Physiology and Pharmacology, 31(2), 1–21.
Garofalo, M., Bennett, A., Farese, A. M., Harper, J., Ward, A., Taylor-Howell, C., et al. (2014). The delayed pulmonary syndrome following acute high-dose irradiation: A rhesus macaque model. Health Physics, 106(1), 56–72.
Ghandhi, S. A., Turner, H. C., Shuryak, I., Dugan, G. O., Bourland, J. D., Olson, J. D., et al. (2018). Whole thorax irradiation of non-human primates induces persistent nuclear damage and gene expression changes in peripheral blood cells. PLoS ONE, 13(1), e0191402.
Ghosh, S. P., Singh, R., Chakraborty, K., Kulkarni, S., Uppal, A., Luo, Y., et al. (2013). Metabolomic changes in gastrointestinal tissues after whole body radiation in a murine model. Molecular BioSystems, 9(4), 723–731.
Golla, S., Golla, J. P., Krausz, K. W., Manna, S. K., Simillion, C., Beyoğlu, D., et al. (2017). Metabolomic analysis of mice exposed to gamma radiation reveals a systemic understanding of total-body exposure. Radiation Research, 187(5), 612–629.
Goudarzi, M., Mak, T. D., Chen, C., Smilenov, L. B., Brenner, D. J., & Fornace, A. J. (2014). The effect of low dose rate on metabolomic response to radiation in mice. Radiation and Environmental Biophysics, 53(4), 645–657.
Green, D. E., & Rubin, C. T. (2014). Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone, 63, 87–94.
Hall, E. J., & Giaccia, A. J. (2018). Radiobiology for the radiologist (8th ed.). Philadelphia: Wolters Kluwer Health.
Hasegawa, A., Ohira, T., Maeda, M., Yasumura, S., & Tanigawa, K. (2016). Emergency responses and health consequences after the Fukushima accident evacuation and relocation. Clinical Oncology, 28(4), 237–244.
Hérodin, F., Valente, M., & Abend, M. (2014). Useful radiation dose biomarkers for early identification of partial-body exposures. Health Physics, 106(6), 750–754.
Hoyles, L., Jiménez-Pranteda, M. L., Chilloux, J., Brial, F., Myridakis, A., Aranias, T., et al. (2018). Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome, 6(1), 73.
Kawamura, K., Qi, F., & Kobayashi, J. (2018). Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. Journal of Radiation Research, 59(Suppl 2), ii91–ii97.
Khan, A. R., Rana, P., Devi, M. M., Chaturvedi, S., Javed, S., Tripathi, R. P., et al. (2011a). Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of γ-irradiated mice. International Journal of Radiation Biology, 87(1), 91–97.
Khan, A. R., Rana, P., Tyagi, R., Kumar, I. P., Devi, M. M., Javed, S., et al. (2011b). NMR spectroscopy based metabolic profiling of urine and serum for investigation of physiological perturbations during radiation sickness. Metabolomics, 7(4), 583–592.
Kultova, G., Tichy, A., Rehulkova, H., & Myslivcova-Fucikova, A. (2020). The hunt for radiation biomarkers: Current situation. International Journal of Radiation Biology, 96(3), 370–382.
Kurland, I. J., Broin, P. Ó., Golden, A., Su, G., Meng, F., Liu, L., et al. (2015). Integrative metabolic signatures for hepatic radiation injury. PLoS ONE, 10(6), e0124795.
Lacombe, J., Sima, C., Amundson, S. A., & Zenhausern, F. (2018). Candidate gene biodosimetry markers of exposure to external ionizing radiation in human blood: A systematic review. PLoS ONE, 13(6), e0198851.
Lee, Y., Pujol Canadell, M., Shuryak, I., Perrier, J. R., Taveras, M., Patel, P., et al. (2018). Candidate protein markers for radiation biodosimetry in the hematopoietically humanized mouse model. Scientific Reports, 8(1), 13557.
Małachowska, B., Tomasik, B., Stawiski, K., Kulkarni, S., Guha, C., Chowdhury, D., et al. (2020). Circulating microRNAs as biomarkers of radiation exposure: A systematic review and meta-analysis. International Journal of Radiation Oncology Biology Physics, 106(2), 390–402.
Meadows, S. K., Dressman, H. K., Daher, P., Himburg, H., Russell, J. L., Doan, P., et al. (2010). Diagnosis of partial body radiation exposure in mice using peripheral blood gene expression profiles. PLoS ONE, 5(7), e11535.
Ozasa, K., Cullings, H. M., Ohishi, W., Hida, A., & Grant, E. J. (2019). Epidemiological studies of atomic bomb radiation at the Radiation Effects Research Foundation. International Journal of Radiation Biology, 95(7), 879–891.
Pannkuk, E. L., Laiakis, E. C., Authier, S., Wong, K., & Fornace, A. J. (2017). Gas chromatography/mass spectrometry metabolomics of urine and serum from nonhuman primates exposed to ionizing radiation: Impacts on the tricarboxylic acid cycle and protein metabolism. Journal of Proteome Research, 16(5), 2091–2100.
Pannkuk, E. L., Laiakis, E. C., Mak, T. D., Astarita, G., Authier, S., Wong, K., et al. (2016). A lipidomic and metabolomic serum signature from nonhuman primates exposed to ionizing radiation. Metabolomics: Official Journal of the Metabolomic Society, 12(5), 80.
Pomper, M. A., & Tarini, G. (2017). Nuclear terrorism—Threat or not? AIP Conference Proceedings, 1898, 050001. https://doi.org/10.1063/1.5009230
Prasanna, P. G. S., Moroni, M., & Pellmar, T. C. (2010). Triage dose assessment for partial-body exposure: Dicentric analysis. Health Physics, 98(2), 244–251.
Rentea, R. M., Lam, V., Biesterveld, B., Fredrich, K. M., Callison, J., Fish, B. L., et al. (2016). Radiation-induced changes in intestinal and tissue-nonspecific alkaline phosphatase: Implications for recovery after radiation therapy. American Journal of Surgery, 212(4), 602–608.
Romano, K. A., Vivas, E. I., Amador-Noguez, D., & Rey, F. E. (2015). Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. American Society for Microbiology, 6(2), e02481.
Shao, L., Luo, Y., & Zhou, D. (2014). Hematopoietic stem cell injury induced by ionizing radiation. Antioxidants & Redox Signaling, 20(9), 1447–1462.
Singh, V. K., Newman, V. L., Romaine, P. L., Hauer-Jensen, M., & Pollard, H. B. (2016). Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Review of Molecular Diagnostics, 16(1), 65–81.
Sproull, M., Kramp, T., Tandle, A., Shankavaram, U., & Camphausen, K. (2017). Multivariate analysis of radiation responsive proteins to predict radiation exposure in total-body irradiation and partial-body irradiation models. Radiation Research, 187(2), 251–258.
Valente, M., Denis, J., Grenier, N., Arvers, P., Foucher, B., Desangles, F., et al. (2015). Revisiting biomarkers of total-body and partial-body exposure in a baboon model of irradiation. PLoS ONE, 10(7), e0132194.
Wang, J., Shao, L., Hendrickson, H. P., Liu, L., Chang, J., Luo, Y., et al. (2015). Total body irradiation in the “hematopoietic” dose range induces substantial intestinal injury in non-human primates. Radiation Research, 184(5), 545–553.
Wang, Z., Klipfell, E., Bennett, B. J., Koeth, R., Levison, B. S., DuGar, B., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 472(7341), 57–63.
Yoshida, T., Goto, S., Kawakatsu, M., Urata, Y., & Li, T. S. (2012). Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radical Research, 46(2), 147–153.
Funding
This work was supported by Defence R & D Organsiation (DRDO), Ministry of Defence, India (INM 313). KM was supported by University Grant Commission (UGC), India.
Author information
Authors and Affiliations
Contributions
PR and RB conceived the project and designed the study, PR supervised the NMR experiments and data analysis. PR, AD and KM involved in experimentation. KM and RT involved in analysis and interpretation of data and writing of manuscript. PR and RB evaluated the manuscript critically and all the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human and/or animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (TIF 5841 kb)
Fig. 1: PCA Loading plots of (PC1 and PC2): (a) Control and TI groups (b) Control and HI groups, (c) Control and AI groups (d) Control, TI, AI and HI groups, (e) Control and TBI groups and (f) Control, TBI and PI groups.
Electronic supplementary material 2 (TIF 3234 kb)
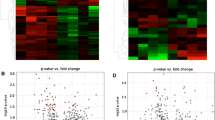
Fig. 2: Multivariate analysis in TI (Thoracic Irradiated) group (a) PCA score plot showing variation between control and TI groups, (b) PLS-DA score plot showing complete separation of metabolic profile of TI from control group. Group segregation is seen in component 1 with 55.7% (elliptical boundary shows 95% confidence interval), (c) VIP plot presenting top 15 metabolites responsible for observed discrimination between the groups, (d) Corresponding Heatmap showing comparison of metabolites between the groups. (C-Red, TI-Green).
Electronic supplementary material 3 (TIF 3595 kb)
Fig. 3: Multivariate analysis in HI (Hind Limb Irradiated) group: (a) PCA score plot showing variation between control and HI groups. (b) PLS-DA score plot showing complete separation of metabolic profile of HI from control group. Group segregation is seen in component 1 with 49.4% (elliptical boundary shows 95% confidence interval). (c) VIP plot presenting top 15 metabolites responsible for observed discrimination between the groups (d) Corresponding Heatmap showing comparison of metabolites between the groups. (C-Red, HI-Green).
Electronic supplementary material 4 (TIF 3282 kb)
Fig. 4: Multivariate analysis in AI (Abdominal region Irradiated) group (a) PCA score plot showing variation between control and AI groups, (b) PLS-DA score plot showing complete separation of metabolic profile of AI from control group. Group segregation is seen in component 1 with 46.8% (elliptical boundary shows 95% confidence interval), (c) VIP plot presenting top 15 metabolites responsible for observed discrimination between the groups, (d) Corresponding Heatmap showing comparison of metabolites between the groups (C-Green, AI-Red).
Electronic supplementary material 5 (TIF 3189 kb)
Fig. 5: Pattern recognition analysis of urine samples (a) PCA score plot showing variation between control, AI, HI and TI groups, (b) PLS-DA score plot showing separation of all irradiated groups from control group (elliptical boundary shows 95% confidence interval) (c) VIP plot presenting top 15 metabolites responsible for observed discrimination between the groups, (d) Corresponding Heatmap showing comparison of metabolites between the groups (C-Green, AI- Red, HI- Purple and TI-Blue).
Electronic supplementary material 6 (TIF 8113 kb)
Fig. 6: Relative levels of peak intensity of significantly perturbed metabolites in all irradiated groups (TI, AI, HI and Pooled Partial) compared to control group. Data is presented as mean ± standard deviation. (*p-value < 0.05, **p-value < 0.01 and ***p-value < 0.001).
Electronic supplementary material 7 (TIF 3422 kb)
Fig. 7: Pattern recognition analysis of urine samples (a) PCA score plot showing variation between control and TBI groups, (b) PLS-DA score plot showing complete separation of metabolic profile of TBI from control group. Group segregation is seen in component 1 with 54% (elliptical boundary shows 95% confidence interval), (c) VIP plot presenting top 15 metabolites are responsible for observed discrimination between the groups, (d) Corresponding Heatmap showing comparison of metabolites between the groups (C-Red, TBI-Green).
Electronic supplementary material 8 (TIF 5557 kb)
Fig. 8: Representative 1H NMR spectrum of urine showing identified metabolites (a) and comparative NMR spectra of control (C) and different irradiated groups (TBI, TI, AI and HI) (b).
Rights and permissions
About this article
Cite this article
Maan, K., Tyagi, R., Dutta, A. et al. Comparative metabolic profiles of total and partial body radiation exposure in mice using an untargeted metabolomics approach. Metabolomics 16, 124 (2020). https://doi.org/10.1007/s11306-020-01742-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-01742-7