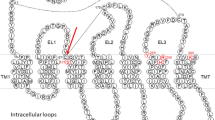
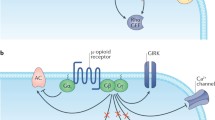
Abstract
G protein-coupled receptors are the target of more than 30% of all FDA-approved drug therapies. Though the purinergic P2 receptors have been an attractive target for therapeutic intervention with successes such as the P2Y12 receptor antagonist, clopidogrel, P2Y2 receptor (P2Y2R) antagonism remains relatively unexplored as a therapeutic strategy. Due to a lack of selective antagonists to modify P2Y2R activity, studies using primarily genetic manipulation have revealed roles for P2Y2R in a multitude of diseases. These include inflammatory and autoimmune diseases, fibrotic diseases, renal diseases, cancer, and pathogenic infections. With the advent of AR-C118925, a selective and potent P2Y2R antagonist that became commercially available only a few years ago, new opportunities exist to gain a more robust understanding of P2Y2R function and assess therapeutic effects of P2Y2R antagonism. This review discusses the characteristics of P2Y2R that make it unique among P2 receptors, namely its involvement in five distinct signaling pathways including canonical Gαq protein signaling. We also discuss the effects of other P2Y2R antagonists and the pivotal development of AR-C118925. The remainder of this review concerns the mounting evidence implicating P2Y2Rs in disease pathogenesis, focusing on those studies that have evaluated AR-C118925 in pre-clinical disease models.


Similar content being viewed by others
Data availability
Not applicable.
References
Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E (2018) Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18(10):601–618
Zuccarini M, Giuliani P, Ronci M, Caciagli F, Caruso V, Ciccarelli R et al (2022) Purinergic signaling in oral tissues. Int J Mol Sci 23(14):7790
Woods LT, Forti KM, Shanbhag VC, Camden JM, Weisman GA (2021) P2Y receptors for extracellular nucleotides: contributions to cancer progression and therapeutic implications. Biochem Pharmacol 187:114406
Volonte C, D’Ambrosi N (2009) Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J 276(2):318–329
Murray JM, Bussiere DE (2009) Targeting the purinome. Methods Mol Biol 575:47–92
Huang Z, Xie N, Illes P, Di Virgilio F, Ulrich H, Semyanov A et al (2021) From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther 6(1):162
Khalafalla MG, Woods LT, Jasmer KJ, Forti KM, Camden JM, Jensen JL et al (2020) P2 Receptors as therapeutic targets in the salivary gland: from physiology to dysfunction. Front Pharmacol 11:222
Kishore SP, Blank E, Heller DJ, Patel A, Peters A, Price M et al (2018) Modernizing the World Health Organization list of Essential Medicines for preventing and controlling cardiovascular diseases. J Am Coll Cardiol 71(5):564–574
Schupke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wohrle J et al (2019) Ticagrelor or Prasugrel in patients with acute coronary syndromes. N Engl J Med 381(16):1524–1534
Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR et al (2020) Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 8(8):775–785
McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J et al (2022) Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 399(10328):909–923
Markham A (2022) Gefapixant: first approval. Drugs 82(6):691–695
Smith JLM, Birring SS, Morice AH, Sher MR, Dicpinigaitis P, Blaiss M, Lanouette S, Harvey L, Yang R, Shaw J, Garin M, Bonuccelli CM (2022) Safety and efficacy of BLU-5937 in the treatment of refractory chronic cough from the phase 2b soothe trial. American Thoracic Society International Conference, San Francisco
Garceau D, Chauret N (2019) BLU-5937: a selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther 56:56–62
Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, Wang X et al (2012) Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol 39(4):720–727
Drill M, Jones NC, Hunn M, O’Brien TJ, Monif M (2021) Antagonism of the ATP-gated P2X7 receptor: a potential therapeutic strategy for cancer. Purinergic Signal 17(2):215–227
Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB, Team DCS (2012) Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis 71(10):1630–1635
Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M et al (2015) Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind. Phase IIa study Inflamm Bowel Dis 21(10):2247–2253
Jacob F, Perez Novo C, Bachert C, Van Crombruggen K (2013) Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal 9(3):285–306
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509(7500):310–317
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461(7261):282–286
Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F, Idzko M (2006) Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J Leukoc Biol 79(1):7–15
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A et al (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314(5806):1792–1795
Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC et al (1994) Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci U S A 91(8):3275–3279
Erb L, Liao Z, Seye CI, Weisman GA (2006) P2 receptors: intracellular signaling. Pflugers Arch 452(5):552–562
Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K et al (2001) An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for G(o)-mediated signal transduction. J Cell Biol 153(3):491–501
Liao Z, Seye CI, Weisman GA, Erb L (2007) The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci 120(Pt 9):1654–1662
Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA et al (2005) The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem 280(47):39050–39057
El-Sayed FG, Camden JM, Woods LT, Khalafalla MG, Petris MJ, Erb L et al (2014) P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells. Am J Physiol Cell Physiol 307(1):C83-96
Siehler S (2009) Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 158(1):41–49
Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H et al (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108(6):809–821
Brown E (2001) Integrin-associated protein (CD47): an unusual activator of G protein signaling. J Clin Invest 107(12):1499–1500
Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI et al (2004) Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279(9):8212–8218
Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279(34):35679–35686
Soltoff SP (1998) Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J Biol Chem. 273(36):23110–7
Yu N, Erb L, Shivaji R, Weisman GA, Seye CI (2008) Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res 102(5):581–588
Woods LT, Jasmer KJ, Munoz Forti K, Shanbhag VC, Camden JM, Erb L et al (2020) P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines. Oral Oncol 109:104808
Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI et al (2010) P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem 285(10):7545–7555
Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI et al (2005) P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem 280(19):18696–18702
Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J et al (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164(5):769–779
Zbodakova O, Chalupsky K, Sarnova L, Kasparek P, Jirouskova M, Gregor M et al (2021) ADAM10 and ADAM17 regulate EGFR, c-Met and TNF RI signalling in liver regeneration and fibrosis. Sci Rep 11(1):11414
Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T et al (2010) The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 65(12):1545–1553
Jasmer KJ, Woods LT, Forti KM, Martin AL, Camden JM, Colonna M et al (2021) P2Y2 receptor antagonism resolves sialadenitis and improves salivary flow in a Sjogren’s syndrome mouse model. Arch Oral Biol 124:105067
Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J et al (2000) P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett 486(3):217–224
Jin J, Dasari VR, Sistare FD, Kunapuli SP (1998) Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol 123(5):789–794
Wang L, Jacobsen SE, Bengtsson A, Erlinge D (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5:16
Gorini S, Callegari G, Romagnoli G, Mammi C, Mavilio D, Rosano G et al (2010) ATP secreted by endothelial cells blocks CX(3)CL 1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y(1)(1) receptor activation. Blood 116(22):4492–4500
Woods LT, Camden JM, Khalafalla MG, Petris MJ, Erb L, Ambrus JL Jr et al (2018) P2Y2 R deletion ameliorates sialadenitis in IL-14α-transgenic mice. Oral Dis 24(5):761–771
Harjunpaa H, Llort Asens M, Guenther C, Fagerholm SC (2019) Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol 10:1078
Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA (2008) P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 [corrected] expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol 45(1):65–75
Keating GM (2015) Diquafosol ophthalmic solution 3%: a review of its use in dry eye. Drugs 75(8):911–922
Moss RB (2013) Pitfalls of drug development: lessons learned from trials of denufosol in cystic fibrosis. J Pediatr 162(4):676–680
Accurso FJ, Moss RB, Wilmott RW, Anbar RD, Schaberg AE, Durham TA et al (2011) Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med 183(5):627–634
Rafehi M, Muller CE (2018) Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol Ther 190:24–80
Xu P, Feng X, Luan H, Wang J, Ge R, Li Z et al (2018) Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorg Med Chem 26(2):366–375
von Kugelgen I (2019) Pharmacology of P2Y receptors. Brain Res Bull 151:12–24
Communi D, Robaye B, Boeynaems JM (1999) Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol 128(6):1199–1206
Pillaiyar T, Funke M, Al-Hroub H, Weyler S, Ivanova S, Schlegel J et al (2020) Design, synthesis and biological evaluation of suramin-derived dual antagonists of the proinflammatory G protein-coupled receptors P2Y2 and GPR17. Eur J Med Chem 186:111789
Jacobson KA, Muller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49
Rafehi M, Malik EM, Neumann A, Abdelrahman A, Hanck T, Namasivayam V et al (2017) Development of potent and selective antagonists for the UTP-activated P2Y4 receptor. J Med Chem 60(7):3020–3038
Kaulich MFS, Mayer R, Müller I, Müller CE (2003) Flavonoids—novel lead compounds for the development of P2Y2 receptor antagonists. Drug Dev Res 59:72–81
Kindon N, Davis A, Dougall I, Dixon J, Johnson T, Walters I et al (2017) From UTP to AR-C118925, the discovery of a potent non nucleotide antagonist of the P2Y2 receptor. Bioorg Med Chem Lett 27(21):4849–4853
Kindon N, Meghani P, Thom SNC (1999) Astrazeneca AB. Novel Compounds. WO1999002501
Mohamady S, Jakeman DL (2005) An improved method for the synthesis of nucleoside triphosphate analogues. J Org Chem 70(25):10588–10591
El-Tayeb A, Qi A, Muller CE (2006) Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem 49(24):7076–7087
El-Tayeb A, Qi A, Nicholas RA, Muller CE (2011) Structural modifications of UMP, UDP, and UTP leading to subtype-selective agonists for P2Y2, P2Y4, and P2Y6 receptors. J Med Chem 54(8):2878–2890
Leeson PD, Springthorpe B (2007) The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov 6(11):881–890
Meghani P (2002) The design of P2Y2 antagonists for the treatment of inflammatory diseases. 224th ACS National Meeting. American Chemical Society, Boston
Kemp PA, Sugar RA, Jackson AD (2004) Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol 31(4):446–455
Rafehi M, Burbiel JC, Attah IY, Abdelrahman A, Muller CE (2017) Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal 13(1):89–103
Henriquez M, Fonseca M, Perez-Zoghbi JF (2018) Purinergic receptor stimulation induces calcium oscillations and smooth muscle contraction in small pulmonary veins. J Physiol 596(13):2491–2506
Perera LMB, Sekiguchi A, Uchiyama A, Uehara A, Fujiwara C, Yamazaki S et al (2019) The regulation of skin fibrosis in systemic sclerosis by extracellular ATP via P2Y2 purinergic receptor. J Invest Dermatol 139(4):890–899
Dai X, Tohyama M, Murakami M, Shiraishi K, Liu S, Mori H et al (2020) House dust mite allergens induce interleukin 33 (IL-33) synthesis and release from keratinocytes via ATP-mediated extracellular signaling. Biochim Biophys Acta Mol Basis Dis 1866(5):165719
Hu LP, Zhang XX, Jiang SH, Tao LY, Li Q, Zhu LL et al (2019) Targeting purinergic receptor P2Y2 prevents the growth of pancreatic ductal adenocarcinoma by inhibiting cancer cell glycolysis. Clin Cancer Res 25(4):1318–1330
Tariba Knezevic P, Vukman R, Uhac M, Illes D, Kovacevic Pavicic D, Simonic-Kocijan S (2020) P2Y2 receptors mediate masseter muscle mechanical hypersensitivity in rats. J Pain Res 13:1323–1333
Magni G, Merli D, Verderio C, Abbracchio MP, Ceruti S (2015) P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia 63(7):1256–1269
Di Virgilio F, Sarti AC, Coutinho-Silva R (2020) Purinergic signaling, DAMPs, and inflammation. Am J Physiol Cell Physiol 318(5):C832–C835
Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ (2004) Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res 317(3):289–298
Khalafalla MG, Woods LT, Camden JM, Khan AA, Limesand KH, Petris MJ et al (2017) P2X7 receptor antagonism prevents IL-1β release from salivary epithelial cells and reduces inflammation in a mouse model of autoimmune exocrinopathy. J Biol Chem 292(40):16626–16637
Di Virgilio F (2007) Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 28(9):465–472
Peterson TS, Thebeau CN, Ajit D, Camden JM, Woods LT, Wood WG et al (2013) Up-regulation and activation of the P2Y(2) nucleotide receptor mediate neurite extension in IL-1β-treated mouse primary cortical neurons. J Neurochem 125(6):885–896
Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI et al (2009) Interleukin-1 β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J Neurochem 109(5):1300–1310
Jin H, Ko YS, Kim HJ (2018) P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer. Int J Oncol 53(5):1953–1966
Baer AN, Hammitt KM (2021) Sjogren’s disease, not syndrome. Arthritis Rheumatol 73(7):1347–1348
Ramos-Casals M, Brito-Zeron P, Bombardieri S, Bootsma H, De Vita S, Dorner T et al (2020) EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann Rheum Dis 79(1):3–18
Mariette X, Criswell LA (2018) Primary Sjogren’s syndrome. N Engl J Med 378(10):931–939
Li L, Jasmer KJ, Camden JM, Woods LT, Martin AL, Yang Y et al (2022) Early dry eye disease onset in a NOD.H-2h4 mouse model of Sjogren’s syndrome. Invest Ophthalmol Vis Sci 63(6):18
Kvarnstrom M, Ottosson V, Nordmark B, Wahren-Herlenius M (2015) Incident cases of primary Sjogren’s syndrome during a 5-year period in Stockholm County: a descriptive study of the patients and their characteristics. Scand J Rheumatol 44(2):135–142
Brandt JE, Priori R, Valesini G, Fairweather D (2015) Sex differences in Sjogren’s syndrome: a comprehensive review of immune mechanisms. Biol Sex Differ 6:19
Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R et al (2012) Primary Sjogren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjogren’s syndrome registry. Arthritis Care Res (Hoboken) 64(6):911–918
van Nimwegen JF, van der Tuuk K, Liefers SC, Verstappen GM, Visser A, Wijnsma RF et al (2020) Vaginal dryness in primary Sjogren’s syndrome: a histopathological case-control study. Rheumatology (Oxford) 59(10):2806–2815
Both T, Dalm VA, van Hagen PM, van Daele PL (2017) Reviewing primary Sjogren’s syndrome: beyond the dryness - from pathophysiology to diagnosis and treatment. Int J Med Sci 14(3):191–200
Nocturne G, Mariette X (2015) Sjogren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 168(3):317–327
Mathews SA, Kurien BT, Scofield RH (2008) Oral manifestations of Sjogren’s syndrome. J Dent Res 87(4):308–318
Mortazavi H, Baharvand M, Movahhedian A, Mohammadi M, Khodadoustan A (2014) Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res 4(4):503–510
Stojan G, Baer AN, Danoff SK (2013) Pulmonary manifestations of Sjogren’s syndrome. Curr Allergy Asthma Rep 13(4):354–360
Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH (2006) Interstitial lung disease in primary Sjogren syndrome. Chest 130(5):1489–1495
Ramos-Casals M, Tzioufas AG, Stone JH, Siso A, Bosch X (2010) Treatment of primary Sjogren syndrome: a systematic review. JAMA 304(4):452–460
McCoy SS, Woodham M, Bunya VY, Saldanha IJ, Akpek EK, Makara MA et al (2022) A comprehensive overview of living with Sjogren’s: results of a National Sjogren’s Foundation survey. Clin Rheumatol 41(7):2071–2078
Martel C, Gondran G, Launay D, Lalloue F, Palat S, Lambert M et al (2011) Active immunological profile is associated with systemic Sjogren’s syndrome. J Clin Immunol 31(5):840–847
Liang Y, Yang Z, Qin B, Zhong R (2014) Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis 73(6):1151–1156
Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J et al (2002) Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum 46(8):2160–2171
Du W, Han M, Zhu X, Xiao F, Huang E, Che N et al (2021) The multiple roles of B Cells in the pathogenesis of Sjogren’s syndrome. Front Immunol 12:684999
Gao Y, Chen Y, Zhang Z, Yu X, Zheng J (2020) Recent advances in mouse models of Sjogren’s syndrome. Front Immunol 11:1158
Shen L, Zhang C, Wang T, Brooks S, Ford RJ, Lin-Lee YC et al (2006) Development of autoimmunity in IL-14α-transgenic mice. J Immunol 177(8):5676–5686
Kayes TD, Weisman GA, Camden JM, Woods LT, Bredehoeft C, Downey EF et al (2016) New murine model of early onset autoimmune thyroid disease/hypothyroidism and autoimmune exocrinopathy of the salivary gland. J Immunol 197(6):2119–2130
Schrader AM, Camden JM, Weisman GA (2005) P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjogren’s syndrome. Arch Oral Biol 50(6):533–40
Ahn JS, Camden JM, Schrader AM, Redman RS, Turner JT (2000) Reversible regulation of P2Y(2) nucleotide receptor expression in the duct-ligated rat submandibular gland. Am J Physiol Cell Physiol 279(2):C286–C294
Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R (2019) Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes 12(6):e005375
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695
Chen X, Qian S, Hoggatt A, Tang H, Hacker TA, Obukhov AG et al (2017) Endothelial cell-specific deletion of P2Y2 receptor promotes plaque stability in atherosclerosis-susceptible ApoE-null mice. Arterioscler Thromb Vasc Biol 37(1):75–83
Hochhauser E, Cohen R, Waldman M, Maksin A, Isak A, Aravot D et al (2013) P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal 9(4):633–642
Khalafalla FG, Greene S, Khan H, Ilves K, Monsanto MM, Alvarez R Jr et al (2017) P2Y2 nucleotide receptor prompts human cardiac progenitor cell activation by modulating hippo signaling. Circ Res 121(11):1224–1236
Ritchie H, Roser M (2017) Obesity. OurWorldInData.org. https://ourworldindata.org/obesity. Accessed 11 Aug 2022
Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ et al (2021) Obesity and cardiovascular eisease: a scientific statement from the American Heart Association. Circulation 143(21):e984–e1010
Franks PW, McCarthy MI (2016) Exposing the exposures responsible for type 2 diabetes and obesity. Science 354(6308):69–73
Kishore B, Zhang Y, Ecelbarger C (2018) US Department of Veterans Affairs. Methods for treating diet-induced obesity by decreasing and inhibiting P2Y2 purinergic receptor expression or activity. US10024846B2
Zhang Y, Ecelbarger CM, Lesniewski LA, Muller CE, Kishore BK (2020) P2Y2 receptor promotes high-fat diet-induced obesity. Front Endocrinol (Lausanne) 11:341
Dusabimana T, Park EJ, Je J, Jeong K, Yun SP, Kim HJ et al (2021) P2Y2R deficiency ameliorates hepatic steatosis by reducing lipogenesis and enhancing fatty acid β-oxidation through AMPK and PGC-1α induction in high-fat diet-fed mice. Int J Mol Sci 22(11):5528
Li W, Wei S, Liu C, Song M, Wu H, Yang Y (2016) Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: the role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med 37(1):63–73
Chang JT (2020) Pathophysiology of inflammatory bowel diseases. N Engl J Med 383(27):2652–2664
Grbic DM, Degagne E, Langlois C, Dupuis AA, Gendron FP (2008) Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol 180(4):2659–2668
Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N et al (2006) ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis 12(8):766–789
Degagne E, Degrandmaison J, Grbic DM, Vinette V, Arguin G, Gendron FP (2013) P2Y2 receptor promotes intestinal microtubule stabilization and mucosal re-epithelization in experimental colitis. J Cell Physiol 228(1):99–109
Stander S (2021) Atopic Dermatitis. N Engl J Med 384(12):1136–1143
Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L et al (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182(6):774–783
Xu H, Wang M, Li Y, Shi M, Wang Z, Cao C et al (2022) Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis 13(5):511
Lu D, Soleymani S, Madakshire R, Insel PA (2012) ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J 26(6):2580–2591
Braun OO, Lu D, Aroonsakool N, Insel PA (2010) Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J Mol Cell Cardiol 49(3):362–369
Chen M, Chen H, Gu Y, Sun P, Sun J, Yu H et al (2021) P2Y2 promotes fibroblasts activation and skeletal muscle fibrosis through AKT, ERK, and PKC. BMC Musculoskelet Disord 22(1):680
Jin H, Seo J, Eun SY, Joo YN, Park SW, Lee JH et al (2014) P2Y2 R activation by nucleotides promotes skin wound-healing process. Exp Dermatol 23(7):480–485
Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani G, Martin MD, Haaksma CJ et al (2006) Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound Repair Regen 14(3):313–320
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3(5):349–363
Parizi M, Howard EW, Tomasek JJ (2000) Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res 254(2):210–220
Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V et al (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308(4):L344–L357
Kagan HM, Li W (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88(4):660–672
Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ (2014) P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 5(19):9322–9334
Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI, Hong SP et al (2009) Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J Exp Clin Cancer Res 28:28
Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR (1999) Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J Biol Chem 274(37):26461–26468
Faria D, Schreiber R, Kunzelmann K (2009) CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch 457(6):1373–1380
Denton CP, Khanna D (2017) Systemic sclerosis. Lancet 390(10103):1685–1699
Kadono T, Kikuchi K, Ihn H, Takehara K, Tamaki K (1998) Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J Rheumatol 25(2):296–301
De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS et al (2013) Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol 40(4):435–446
Kitaba S, Murota H, Terao M, Azukizawa H, Terabe F, Shima Y et al (2012) Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol 180(1):165–176
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 378(19):1811–1823
Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA et al (2016) Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J 47(1):243–253
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U et al (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370(22):2071–2082
Richeldi L, Azuma A, Cottin V, Hesslinger C, Stowasser S, Valenzuela C et al (2022) Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med 386(23):2178–2187
Muller T, Fay S, Vieira RP, Karmouty-Quintana H, Cicko S, Ayata K et al (2017) The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease. Oncotarget 8(22):35962–35972
Burnstock G, Evans LC, Bailey MA (2014) Purinergic signalling in the kidney in health and disease. Purinergic Signal 10(1):71–101
Menzies RI, Tam FW, Unwin RJ, Bailey MA (2017) Purinergic signaling in kidney disease. Kidney Int 91(2):315–323
Verschuren EHJ, Rigalli JP, Castenmiller C, Rohrbach MU, Bindels RJM, Peters DJM et al (2020) Pannexin-1 mediates fluid shear stress-sensitive purinergic signaling and cyst growth in polycystic kidney disease. FASEB J 34(5):6382–6398
Satoskar AA, Parikh SV, Nadasdy T (2020) Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat Rev Nephrol 16(1):32–50
Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C (2020) Lupus nephritis. Nat Rev Dis Primers 6(1):7
DeVrieze BW, Hurley JA (2022) Goodpasture syndrome. StatPearls, Treasure Island (FL)
Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB et al (2016) IgA nephropathy. Nat Rev Dis Primers 2:16001
Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, Cavazzana I et al (2001) Clinical and morphological features of kidney involvement in primary Sjogren’s syndrome. Nephrol Dial Transplant 16(12):2328–2336
Maripuri S, Grande JP, Osborn TG, Fervenza FC, Matteson EL, Donadio JV et al (2009) Renal involvement in primary Sjogren’s syndrome: a clinicopathologic study. Clin J Am Soc Nephrol 4(9):1423–1431
Centers for Disease Control and Prevention (2021) Chronic kidney disease in the United States, 2021. US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta
Rennert L, Zschiedrich S, Sandner L, Hartleben B, Cicko S, Ayata CK et al (2018) P2Y2R signaling is involved in the onset of glomerulonephritis. Front Immunol 9:1589
Remuzzi G, Schieppati A, Ruggenenti P (2002) Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med 346(15):1145–51
Furman BL (2021) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 1(4):e78
Tan RZ, Zhong X, Li JC, Zhang YW, Yan Y, Liao Y et al (2019) An optimized 5/6 nephrectomy mouse model based on unilateral kidney ligation and its application in renal fibrosis research. Ren Fail 41(1):555–566
Kim H, Dusabimana T, Kim SR, Je J, Jeong K, Kang MC et al (2018) Supplementation of Abelmoschus manihot ameliorates diabetic nephropathy and hepatic steatosis by activating autophagy in mice. Nutrients 10(11):1703
Dusabimana T, Kim SR, Park EJ, Je J, Jeong K, Yun SP et al (2020) P2Y2R contributes to the development of diabetic nephropathy by inhibiting autophagy response. Mol Metab 42:101089
Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N (2020) Autophagy in diabetic nephropathy: a review. Int Urol Nephrol 52(9):1705–1712
Zaparte A, Cappellari AR, Brandao CA, de Souza JB, Borges TJ, Kist LW et al (2021) P2Y2 receptor activation promotes esophageal cancer cells proliferation via ERK1/2 pathway. Eur J Pharmacol 891:173687
Leemans CR, Snijders PJF, Brakenhoff RH (2018) The molecular landscape of head and neck cancer. Nat Rev Cancer 18(5):269–282
Chow LQM (2020) Head and Neck Cancer. N Engl J Med 382(1):60–72
Taberna M, Oliva M, Mesia R (2019) Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol 9:383
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y et al (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382(1):41–50
Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL (2004) Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22(1):77–85
Cassell A, Grandis JR (2010) Investigational EGFR-targeted therapy in head and neck squamous cell carcinoma. Expert Opin Investig Drugs 19(6):709–722
Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D et al (2009) Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 27(11):1864–1871
Grandis JR, Tweardy DJ (1993) Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53(15):3579–3584
Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J (2008) Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 112(12):2710–2719
Quesnelle KM, Wheeler SE, Ratay MK, Grandis JR (2012) Preclinical modeling of EGFR inhibitor resistance in head and neck cancer. Cancer Biol Ther 13(10):935–945
Burnstock G, Vaughn B, Robson SC (2014) Purinergic signalling in the liver in health and disease. Purinergic Signal 10(1):51–70
Xie R, Xu J, Wen G, Jin H, Liu X, Yang Y et al (2014) The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem 289(27):19137–19149
Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M et al (2012) Purinergic P2Y(2) receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 143(6):1620–9.e4
Velazquez-Miranda E, Molina-Aguilar C, Gonzalez-Gallardo A, Vazquez-Martinez O, Diaz-Munoz M, Vazquez-Cuevas FG (2020) Increased purinergic responses dependent on P2Y2 receptors in hepatocytes from CCl4-treated fibrotic mice. Int J Mol Sci 21(7):2305
Tackett BC, Sun H, Mei Y, Maynard JP, Cheruvu S, Mani A et al (2014) P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 307(11):G1073–G1087
Schulien I, Hockenjos B, van Marck V, Ayata CK, Follo M, Thimme R et al (2020) Extracellular ATP and purinergic P2Y2 receptor signaling promote liver tumorigenesis in mice by exacerbating DNA damage. Cancer Res 80(4):699–708
Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M et al (2006) c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev 20(16):2306–2314
Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV (2020) Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 12(2):173–181
Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM (1999) NF-kB/Rel activation in cerulein pancreatitis. Gastroenterology 116(2):420–430
Ferreira RMM, Sancho R, Messal HA, Nye E, Spencer-Dene B, Stone RK et al (2017) Duct- and acinar-derived pancreatic ductal adenocarcinomas show distinct tumor progression and marker expression. Cell Rep 21(4):966–978
Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ (1995) Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 376(6540):517–519
Gho YS, Kim PN, Li HC, Elkin M, Kleinman HK (2001) Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res 61(10):4253–4257
Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC et al (2014) P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res 16(5):R77
Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX et al (2013) P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer 109(6):1666–1675
Weisman GA, Camden JM, Peterson TS, Ajit D, Woods LT, Erb L (2012) P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y(2) receptor interactions in neuroinflammation. Mol Neurobiol 46(1):96–113
Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C et al (2004) P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 127(2):431–441
Rodriguez-Zayas AE, Torrado AI, Miranda JD (2010) P2Y2 receptor expression is altered in rats after spinal cord injury. Int J Dev Neurosci 28(6):413–421
Franke H, Illes P (2006) Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther 109(3):297–324
Inoue K (2008) Purinergic systems in microglia. Cell Mol Life Sci 65(19):3074–3080
Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY et al (2010) P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol 41(2–3):356–366
Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A et al (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 3(125):ra45
Messlinger K, Russo AF (2019) Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 39(13):1661–1674
Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M et al (2011) Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci. 31(10):3638–49
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179(2):312–339
Onuska KM (2020) The dual role of microglia in the progression of Alzheimer’s disease. J Neurosci 40(8):1608–1610
Passamonti L, Tsvetanov KA, Jones PS, Bevan-Jones WR, Arnold R, Borchert RJ et al (2019) Neuroinflammation and functional connectivity in Alzheimer’s disease: interactive influences on cognitive performance. J Neurosci 39(36):7218–7226
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217(2):459–472
Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M (2016) TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91(2):328–340
Sosna J, Philipp S, Albay R 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM et al (2018) Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13(1):11
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS et al (2018) IL-1beta, IL-6, TNF-α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep 8(1):12050
Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Gibson Wood W et al (2012) Nucleotides released from Aβ1-42-treated microglial cells increase cell migration and Aβ1-42 uptake through P2Y2 receptor activation. J Neurochem 121(2):228–238
Ajit D, Woods LT, Camden JM, Thebeau CN, El-Sayed FG, Greeson GW et al (2014) Loss of P2Y2 nucleotide receptors enhances early pathology in the TgCRND8 mouse model of Alzheimer’s disease. Mol Neurobiol 49(2):1031–1042
Eberhardt N, Bergero G, Mazzocco Mariotta YL, Aoki MP (2022) Purinergic modulation of the immune response to infections. Purinergic Signal 18(1):93–113
Vanderstocken G, Van de Paar E, Robaye B, di Pietrantonio L, Bondue B, Boeynaems JM et al (2012) Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS ONE 7(11):e50385
Chen B (2019) Molecular mechanism of HIV-1 entry. Trends Microbiol 27(10):878–891
Barmania F, Pepper MS (2013) C-C chemokine receptor type five (CCR5): an emerging target for the control of HIV infection. Appl Transl Genom 2:3–16
Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR (1997) The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A 94(5):1925–1930
Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H et al (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med 208(9):1823–1834
D’Amico D, Valdebenito S, Eugenin EA (2021) The role of pannexin-1 channels and extracellular ATP in the pathogenesis of the human immunodeficiency virus. Purinergic Signal 17(4):563–576
Zhang M, Piskuric NA, Vollmer C, Nurse CA (2012) P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. J Physiol 590(17):4335–4350
Horioka M, Ceraudo E, Lorenzen E, Sakmar TP, Huber T (2021) Purinergic receptors crosstalk with CCR5 to amplify Ca2+ signaling. Cell Mol Neurobiol 41(5):1085–1101
Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S et al (2013) Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 11(8):539–550
Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER (2005) Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent αvβ3 integrin conformers. Proc Natl Acad Sci U S A 102(4):1163–1168
Bondu V, Wu C, Cao W, Simons PC, Gillette J, Zhu J et al (2017) Low-affinity binding in cis to P2Y2R mediates force-dependent integrin activation during hantavirus infection. Mol Biol Cell 28(21):2887–2903
Bondu V, Bitting C, Poland VL, Hanson JA, Harkins MS, Lathrop S et al (2018) Upregulation of P2Y2R, active uPA, and PAI-1 are essential components of hantavirus cardiopulmonary syndrome. Front Cell Infect Microbiol 8:169
Sévigny J, Mabrouka S (2018) Université Laval. Treatment of inflammatory bowel disease. WO2018058246A1
Gabrilovich DI (2020) The Wistare Institute of Anatomy and Biology. Compositions and methods for altering neutrophil migration and metastasis. WO2020081452
Conroy S, Kindon ND, Glenn J, Stoddart LA, Lewis RJ, Hill SJ et al (2018) Synthesis and evaluation of the first fluorescent antagonists of the human P2Y2 receptor based on AR-C118925. J Med Chem 61(7):3089–3113
Funding
This work was supported by National Institute of Dental and Craniofacial Research grants R01DE007389 (GAW), R01DE023342 (GAW), R01DE029833 (GAW, SC, KJJ), and R21AR079693 (SC) and a Sjögren’s Foundation grant (KJJ, SC). KMF is supported by a University of Missouri Life Sciences Fellowship and the Wayne L. Ryan Foundation Fellowship from the Ryan Foundation.
Author information
Authors and Affiliations
Contributions
All authors developed the idea for this review. KJJ and KMF performed literature searches, KJJ and KMF drafted the manuscript, and KJJ, LTW, SC, and GAW critically revised the draft.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jasmer, K.J., Muñoz Forti, K., Woods, L.T. et al. Therapeutic potential for P2Y2 receptor antagonism. Purinergic Signalling 19, 401–420 (2023). https://doi.org/10.1007/s11302-022-09900-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09900-3