Abstract
Atractylenolide I (Atr-I) was found to sensitize a variety of human cancer cells in previous studies. Purinergic P2X7R plays important role in different cancers. However, whether Atr-I could generate antitumor activity in human cervical cancer cells and P2X7R get involved in this effect remain unclear. In this study, Hela (HPV 18 +) and SiHa (HPV 16 +) cells were treated with different doses of Atr-I. The results indicated that agonist and antagonist of P2X7 receptors, BzATP and JNJ-47965567 (JNJ), could suppress the proliferation of Hela and SiHa cells. Atr-I demonstrated a considerable antitumor effect in both human cervical cancer cells in vitro. Atr-I combined with P2X7R agonist, BzATP, restored Atr-I-induced growth inhibition in Hela cells but not in SiHa cells. However, the combinatorial treatment of P2X7R antagonist JNJ and Atr-I has an additive effect on cell growth inhibition in SiHa cells rather than in Hela cells. It implied that P2X7R would get involved in the anti-human cervical cancer cells effect of Atr-I.
Similar content being viewed by others
Introduction
Cervical cancer is the fourth most common cause of morbidity and mortality among women with gynaecologic malignancies worldwide, and there were an estimated 600,000 new cases and 340,000 deaths in 2020 [1]. A large majority of cervical cancer (more than 95%) is due to the human papillomavirus (HPV) and HPV types 16 and 18 cause at least 70% of cervical cancers [2]. In the past few decades, despite the application of human papillomavirus (HPV) vaccine has been proven to dramatically reduce the risk of cervical cancer, the fact which cannot be neglected revealed that a high proportion of cervical cancer cases were clinically diagnosed. The surgical or radiotherapeutic-treated cases still suffer from recurrence or metastasis, which give rise to poor clinical outcomes and prognosis [3,4,5]. It is still a big challenge to find out new promising target or potential new drug for the treatment of cervical cancer [6, 7].
In recent years, purinergic signaling has been recognized to be a promising target in a variety of diseases in the whole body [8,9,10] since it was proposed by Burnstock in 1972 [11]. In the purinergic system, growing evidence supported that purinergic P2X7Rs play important role in different cancers, including lung cancer, colorectal cancer, breast cancer, and acute myeloid leukemia [12,13,14,15,16,17,18,19]. Apart from the strategy targeting the P2X7 ion channel, from antibodies and nanobodies to small-molecule drugs, which has been proved to improve the outcomes in models of cancer [20,21,22,23,24], the nature product is also taken into account for the development of potential anti-cancer drug to target purinergic receptors [25, 26]. Atractylenolide I (Atr-I, Fig. 1A), a natural product extracted from Atractylodes macrocephala Koidz, was found to sensitize human colorectal cancer cells, ovarian cancer cells, breast cancer, gastric cancer, and bladder cancer cells in previous studies [27,28,29,30,31,32,33,34]. However, whether Atr-I would be able to raise antitumor activity in human cervical cancer or P2X7 receptors would be involved in the antitumor activity of Atr-I remains unclear. Hence, this study aims to obtain the in vitro evidence of antitumor activity and the role of P2X7 receptors in Atr-I-treated human cervical cancer cells.
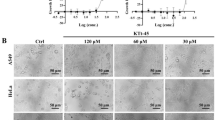
Atractylenolide I inhibits human cervical cancer cells’ proliferation in vitro. (A) Chemical structure of atractylenolide I. (B) The CCK8 assay of Hela cells and SiHa cells treated with the designed concentration of atractylenolide I for 24 h, 48 h, and 72 h. (C) LDH release assay in cells after exposure to increased atractylenolide I concentration for 24 h, 48 h, and 72 h. The colony formation assay of Hela cells and SiHa cells incubated with the increased atractylenolide I concentration for 2 weeks. The characteristic images (D) and the quantification of colonies (E). (F) The protein of P2X7R in Hela cells and SiHa cells was detected by Western blot after 48 h of the treatment with atractylenolide I and the quantification of the protein of P2X7R (G). All data are means ± SD. *p < 0.05, **p < 0.01,***p < 0.001 as compared with the atractylenolide I-untreated group
Materials and methods
Cell culture
Human cervical cancer cell lines (Hela, HPV 18 + ; SiHa, HPV 16 +) were obtained from the ATCC and cultured in DMEM supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, USA), 100U/ml penicillin (Thermo Fisher Scientific, USA), and 100U/ml streptomycin (Thermo Fisher Scientific, USA), at 37 °C in 5% CO2.
Antibodies and reagents
Antibodies were purchased from Proteintech (GAPDH Monoclonal antibody 60,004–1-Ig, HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H + L) SA00001-2, HRP-conjugated Affinipure Goat Anti-Mouse IgG(H + L) SA00001-1) or Abcam (P2X7R ab93354, UK). The specific agonist to P2X7R, 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP, B6396) was purchased from Sigma. The selective P2X7 antagonist JNJ-47965567 (JNJ) was purchased from Tocris Bioscience (Bristol, UK). Atr-I (73,069–13-3) was purchased from Herbpurify (Chengdu, China).
Cell proliferation inhibition test
Cell Counting Kit-8 (CCK-8, AbMole, USA) and colony formation assays were used to detect cell proliferation. Briefly, the cells used for CCK-8 assay were inoculated (1 × 106 cells/ml) into a 96-well plate, followed by the addition of 100 µl of cell suspension to each well and received the designed concentrations of drug treatment. The detailed procedures have been described by Bai et al. [35]. The cells for colony formation assay were cultured in 24-well plates (800 cells/well) and incubated with the designed treatments. After 2 weeks, the 4% paraformaldehyde was adopted for fixation and crystal violet was used for staining. The visible colonies were calculated applying ImageJ software.
Western blot analysis
Cells were lysed in ice with RIPA buffer (150 mmol/l Tris–HCl pH 7.0, 150 mmol/l NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1%SDS) containing with protease inhibitor cocktail (Roche, 4,693,159,001) for 30 min. The Bio-Rad protein assay was used to quantify the lysates. The protein samples were subjected to NuPAGE™ 10% Bis–Tris Protein Gels (Invitrogen, NP0302BOX) and probed with the indicated antibodies to visualize the protein levels using a ChemiScope 6000 Touch.
Lactate dehydrogenase release assay
Lactate dehydrogenase (LDH) test kit (Beyotime Biotechnology, Shanghai, China) was used to assess the cytotoxicity of atractylenolide I and in combination with or without 100 μM BzATP or 20 μM JNJ. Cells were cultured in 96-well plates (6 × 103cells/well). After treatment with the designed concentrations of drugs, cell culture supernatant was transferred to the new 96-well plate for LDH analysis.
Flow cytometry
The ratio of apoptotic cells was measured with an Annexin V-AF647/PI Apoptosis Kit (E-CK-A213; Elabscience biotechnology, Wuhan, China) according to the manufacturer’s instructions. Cells were harvested and washed twice with PBS and then resuspended in 500 μl binding buffer. After adding 5 µl annexinV-AF647 and 5 μl PI into the cell suspension, respectively, at least 20,000 live cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed by using FlowJo software (FlowJo, Ashland, OR, USA).
Statistical analysis
All experiments were performed at least three times independently. All statistical analysis and graphics were performed using GraphPad Prism 7.0 Software (GraphPad Software Inc., San Diego, CA). For two-group comparisons, Student’s two-tailed t-test was used. For multiple group comparisons, one-way ANOVA was used. A value of P < 0.05 was considered statistically significant.
Results
Atractylenolide I represses human cervical cancer cells’ growthin vitro
To evaluate whether Atr-I exhibits an antitumor effect against human cervical cancer cells, Hela cells and SiHa cells were treated with different doses of Atr-I. CCK8 assay showed that Atr-I markedly inhibited the growth of Hela cells and SiHa cells in a dose- and time-dependent manner. Notably, Hela cells were highly sensitive to Atr-I, whereas SiHa cells demonstrated higher tolerance to Atr-I (Fig. 1B). Consistently, the proliferation of Hela cells was significantly inhibited than that of SiHa cells in response to Atr-I treatment, as evidenced by reduced colony formation (Fig. 1D, E). Then, we performed LDH release assay and found that Atr-I damaged the integrity of the plasma membrane, which was most obvious at 72 h after treatment (Fig. 1C). Taken together, these results suggest that Atr-I demonstrate a considerable antitumor effect in human cervical cancer cells in vitro.
The antitumor activity of atractylenolide I involves the P2X7 receptor in human cervical cancer cells
Next, we intended to evaluate whether P2X7 receptor was involved in the anti-human cervical cancer cells’ effect of Atr-I. Firstly, the western blot analysis indicated that the decreased expression of P2X7 receptor protein was observed in Atr-I-treated Hela cells and SiHa cells (Fig. 1F, G). And then, CCK8 assay showed that BzATP, the selective agonist of P2X7 receptor, and JNJ, a selective small-molecule antagonist of P2X7 receptor, markedly inhibited the growth of Hela cells and SiHa cells with a dose- and time-dependent manner (Fig. 2A, Fig. 3A). Interestingly, SiHa cells are more sensitive to BzATP. When human cervical cancer cells were treated with BzATP or JNJ and Atr-I together, the data indicated that BzATP partially restored Atr-I-induced growth inhibition in Hela cells, but it was hardly affected in SiHa cells (Fig. 2B). Consistently, the LDH release of Hela cells was significantly inhibited in response to combinational treatment of BzATP (Fig. 2C), as evidenced by increased colony formation (Fig. 2D, E). Whereas the combinatorial treatment of JNJ and Atr-I has an additive effect on cell growth inhibition in SiHa and Hela cells (Fig. 3B). Consistently, the LDH release of SiHa cells was significantly elevated in response to combinational treatment of JNJ (Fig. 3C) after 48 h, as evidenced by reduced colony formation (Fig. 3D, E). Finally, the data from flow cytometry presented that BzATP partially restored Atr-I-induced apotosis in Hela cells but not in SiHa cells (Fig. 2F, G). Whereas the combinatorial treatment of JNJ and Atr-I, JNJ partially promoted Atr-I-induced apotosis in SiHa cells but not in Hela cells (Fig. 3F, G). Taken together, these results implied that the P2X7 receptor was involved in the anti-human cervical cancer cells effect of Atr-I with different roles in HeLa cells and SiHa cells.
Activation of P2X7R affects cervical carcinoma cells’ proliferation. (A) The CCK8 assay of Hela cells and SiHa cells treated with the designed concentration of BzATP for 24 h, 48 h, and 72 h. (B) CCK8 assay of Hela cells and SiHa cells indicated the presence or absence of atractylenolide I and in combination with or without 100 μM BzATP for 24 h, 48 h, and 72 h. (C) LDH release assay in cells treated as in B. The colony formation assay in cells incubated under these same conditions for 2 weeks. The characteristic images (D) and the quantification of colonies (E). (F, G) Hela cells and SiHa cells treated with atractylenolide I or 100 μM BzATP alone or their mixture for 48 h were subjected to flow cytometry analysis. All data are means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Inhibition of P2X7R affects cervical carcinoma cells’ proliferation. (A) The CCK8 assay of Hela cells and SiHa cells treated with the designed concentration of JNJ for 24 h, 48 h, and 72 h. (B) CCK8 assay of Hela cells and SiHa cells indicated the presence or absence of atractylenolide I and in combination with or without 20 μM JNJ for 24 h, 48 h, and 72 h. (C) LDH release assay in cells treated as in B. The colony formation assay in cells incubated under these same conditions for 2 weeks. The characteristic images (D) and the quantification of colonies (E). (F, G) Hela cells and SiHa cells treated with atractylenolide I or 20 μM JNJ alone or their mixture for 48 h were subjected to flow cytometry analysis. All data are means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
In this study, we firstly found that Atr-I generated a considerable antitumor effect in 2 different HPV phenotypes of human cervical cancer cell lines (Hela, HPV 18 + ; SiHa, HPV 16 +) in vitro. It supports that Atr-I would be a potential promising anti-cancer compound for the treatment of human cervical cancer, especially caused by HPV 18 and HPV 16, as well as colorectal, ovarian, breast, gastric, and bladder cancers [27,28,29,30,31,32,33,34]. However, due to the fact that in the current study Hela cells were highly sensitive to Atr-I, whereas SiHa cells demonstrated higher tolerance to Atra-I, it implied that we cannot neglect that the different cell lines would be the reason to present different responses to Atr-I treatment. Additional cell lines of human cervical cancer, including Bu25TK, CaSki, MS751, C33A, Me180, and HT-3, or in vivo data is needed to confirm the antitumor effect of Atr-I.
Based on current data, it seems that the role of purinergic P2X7R in the antitumor effect of Atr-I is diversity. The reduced protein expression of P2X7 receptor in both Hela and SiHa cells was observed in this study. It implied that P2X7R might be able to get involved in the antitumor mechanism of Atr-I in 2 different human cervical cell lines. In Hela cells, co-application of P2X7 agonist BzATP and Atr-I led to the decreased antitumor effect whereas no changes were found under the condition combination of P2X7 antagonist JNJ and Atr-I. It suggested that the antitumor effect of Atr-I in Hela cells with HPV 18 positive would be linked with P2X7 receptor. However, it would be another possibility in SiHa cells with HPV 16 positive. Co-administration of P2X7 antagonist JNJ rather than the agonist BzATP and Atr-I raised the antitumor effect although the single use of both agonist of P2X7 BzATP and the antagonist JNJ could generate antitumor effect in Hela and Siha cells. It could be proposed that the antitumor effect of Atr-I in Siha cells would be associated with purinergic P2X7 receptors and its downstream pathway (PI3K/Akt/GSK-3β, AKT, AMPK-PRAS-40-mTOR, NOD-like receptor containing a pyrin inflammasome, etc.) which is regarded as the underlying mechanism of P2X7 receptors in cancers [36,37,38,39].
It is worth pointing out that in our study both BzATP and JNJ have antitumor effect in Hela and SiHa cells. BzATP is the agonist to active P2X7 receptor, theoretically which would be harmful for the treatment of cancer in that increasing evidence demonstrated that suppression of high concentration of ATP in tumor microenvironment and the activation of P2X7 is recognized as a promising strategy for the treatment of different tumors [12, 18, 20, 38, 39]. However, ATP is considered a double-edged sword in cancer [40, 41] and BzATP could inhibit breast cancer cell migration [42]. It indicated that both the agonist (BzATP) and antagonist (JNJ) of P2X7 receptor would be useful to inhibit the proliferation of human cervical cancer. Maybe the reason would be related to the complicated regulation of downstream signaling pathways by P2X7, including the concentration and duration of agonist exposure, the interaction of P2X7 with other receptors, and the interaction with the actin cytoskeleton machinery [38, 39, 43,44,45]. On the other hand, co-administration of P2X7 antagonist JNJ rather than the agonist BzATP and Atr-I raised the antitumor effect, which might be derived from the complicated microenvironment of human cervical cancer. More comprehensive evidence would be pursued in the future design and research.
Conclusions
This study demonstrated that both the agonist and antagonist of P2X7 receptors, BzATP and JNJ, could suppress the proliferation of Hela and SiHa cells. And the P2X7 receptor would get involved in the antitumor effect of Atr-I in the 2 different human cervical cancer cell lines (HPV 18 + in hela and HPV 16 + in SiHa) with different HPV phenotypes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS (2018) Screening for cervical cancer with high-risk human papillomavirus testing: Updated evidence report and systematic review for the US preventive services task force. JAMA 320(7):687–705. https://doi.org/10.1001/jama.2018.10400
Nama V, Angelopoulos G, Twigg J, Murdoch JB, Bailey J, Lawrie TA (2018) Type II or type III radical hysterectomy compared to chemoradiotherapy as a primary intervention for stage IB2 cervical cancer. Cochrane Database Syst Rev 10(10):CD011478. https://doi.org/10.1002/14651858.CD011478.pub2
Simms KT, Steinberg J, Caruana M et al (2019) Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol 20(3):394–407. https://doi.org/10.1016/S1470-2045(18)30836-2
Wendel Naumann R, Leath CA 3rd (2020) Advances in immunotherapy for cervical cancer. Curr Opin Oncol 32(5):481–487. https://doi.org/10.1097/CCO.0000000000000663
Sharma S, Deep A, Sharma AK (2020) Current Treatment for Cervical Cancer: An Update. Anticancer Agents Med Chem 20(15):1768–1779. https://doi.org/10.2174/1871520620666200224093301
Burnstock G (2018) The therapeutic potential of purinergic signalling. Biochem Pharmacol 151:157–165. https://doi.org/10.1016/j.bcp.2017.07.016
Burnstock G (2017) Purinergic Signalling: Therapeutic Developments Front Pharmacol 8:661. https://doi.org/10.3389/fphar.2017.00661
Huang Z, Xie N, Illes P et al (2021) From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther 6(1):162. https://doi.org/10.1038/s41392-021-00553-z
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24(3):509–581
Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E (2018) Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18(10):601–618. https://doi.org/10.1038/s41568-018-0037-0
Li Q, Zhu X, Song W, Peng X, Zhao R (2020) The P2X7 purinergic receptor: a potential therapeutic target for lung cancer. J Cancer Res Clin Oncol 146(11):2731–2741. https://doi.org/10.1007/s00432-020-03379-4
Calik I, Calik M, Turken G, Ozercan IH (2020) A promising independent prognostic biomarker in colorectal cancer: P2X7 receptor. Int J Clin Exp Pathol 13(2):107–121
Calik I, Calik M, Sarikaya B, Ozercan IH, Arslan R, Artas G, Dagli AF (2020) P2X7 receptor as an independent prognostic indicator in gastric cancer. Bosn J Basic Med Sci 20(2):188–196. https://doi.org/10.17305/bjbms.2020.4620
Zhang Y, Ding J, Wang L (2019) The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci 64(2):388–394. https://doi.org/10.1016/j.advms.2019.05.002
Zheng L, Zhang X, Yang F, Zhu J, Zhou P, Yu F, Hou L, Xiao L, He Q, Wang B (2014) Regulation of the P2X7R by microRNA-216b in human breast cancer. Biochem Biophys Res Commun 452(1):197–204. https://doi.org/10.1016/j.bbrc.2014.07.101
Vultaggio-Poma V, Sarti AC, Di Virgilio F (2020) Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 9(11):2496. https://doi.org/10.3390/cells9112496
Pegoraro A, Adinolfi E (2021) The ATP/P2X7 axis is a crucial regulator of leukemic initiating cells proliferation and homing and an emerging therapeutic target in acute myeloid leukemia. Purinergic Signalling 17:319–321. https://doi.org/10.1007/s11302-021-09789-4
Burnstock, G., Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signalling.2018;1–18. https://doi.org/10.1007/s11302-017-9593-0
Zhang WJ, Hu CG, Zhu ZM, Luo HL (2020) Effect of P2X7 receptor on tumorigenesis and its pharmacological properties. Biomed Pharmacother 125:109844. https://doi.org/10.1016/j.biopha.2020.109844
Koch-Nolte F, Eichhoff A, Pinto-Espinoza C et al (2019) Novel biologics targeting the P2X7 ion channel. Curr Opin Pharmacol 47:110–118. https://doi.org/10.1016/j.coph.2019.03.001
Sarti AC, Vultaggio-Poma V. & Di Virgilio F. P2X7: a receptor with a split personality that raises new hopes for anti-cancer therapy. Purinergic Signalling.2021;175–178. doi.org/https://doi.org/10.1007/s11302-021-09783-w
Douguet L, Janho Dit Hreich S, Benzaquen J, et al. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat Commun. 2021;12(1):653. https://doi.org/10.1038/s41467-021-20912-2
Jelassi B, Anchelin M, Chamouton J et al (2013) Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 34(7):1487–1496. https://doi.org/10.1093/carcin/bgt099
Ai X, Dong X, Guo Y et al (2021) Targeting P2 receptors in purinergic signaling: a new strategy of active ingredients in traditional Chinese herbals for diseases treatment. Purinergic Signal 17(2):229–240. https://doi.org/10.1007/s11302-021-09774-x
Wang K, Huang W, Sang X et al (2020) Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 68:153191. https://doi.org/10.1016/j.phymed.2020.153191
Long F, Lin H, Zhang X, Zhang J, Xiao H, Wang T (2020) Atractylenolide-I Suppresses Tumorigenesis of Breast Cancer by Inhibiting Toll-Like Receptor 4-Mediated Nuclear Factor-κB Signaling Pathway. Front Pharmacol 11:598939. https://doi.org/10.3389/fphar.2020.598939
Wang K, Huang W, Sang X, Wu X, Shan Q, Tang D, Xu X, Cao G (2020) Atractylenolide I inhibits colorectal cancer cell proliferation by affecting metabolism and stemness via AKT/mTOR signaling. Phytomedicine 68:153191. https://doi.org/10.1016/j.phymed.2020.153191
Li Y, Wang Y, Liu Z, Guo X, Miao Z, Ma S (2020) Atractylenolide I Induces Apoptosis and Suppresses Glycolysis by Blocking the JAK2/STAT3 Signaling Pathway in Colorectal Cancer Cells. Front Pharmacol 11:273. https://doi.org/10.3389/fphar.2020.00273
Long F, Wang T, Jia P, Wang H, Qing Y, Xiong T, He M, Wang X (2017) Anti-Tumor Effects of Atractylenolide-I on Human Ovarian Cancer Cells. Med Sci Monit 23:571–579. https://doi.org/10.12659/msm.902886
Ma L, Mao R, Shen K, Zheng Y, Li Y, Liu J, Ni L (2014) Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem Biophys Res Commun 450(1):353–359. https://doi.org/10.1016/j.bbrc.2014.05.110
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng Y, Ma Q (2016) Anti-tumor effects of Atractylenolide I on bladder cancer cells. J Exp Clin Cancer Res 35:40. https://doi.org/10.1186/s13046-016-0312-4
Chan KWK, Chung HY, Ho WS (2020) Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro. Molecules 25(1):212. https://doi.org/10.3390/molecules25010212
Bai C Zhang Z Zhou L Zhang HY Chen Y Tang Y. Repurposing Ziyuglycoside II Against Colorectal Cancer via Orchestrating Apoptosis and Autophagy. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.576547
Zhang WJ, Luo C, Huang C, Pu FQ, Zhu JF, Zhu ZM (2021) PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur J Pharmacol 899:174041. https://doi.org/10.1016/j.ejphar.2021.174041
Zhu X, Li Q, Song W, Peng X, Zhao R (2021) P2X7 receptor: a critical regulator and potential target for breast cancer. J Mol Med (Berl) 99(3):349–358. https://doi.org/10.1007/s00109-021-02041-x
Lara R, Adinolfi E, Harwood CA et al (2020) P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front Pharmacol 11:793. https://doi.org/10.3389/fphar.2020.00793
Bian S, Sun X, Bai A et al (2013) P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One 8(4):e60184. https://doi.org/10.1371/journal.pone.0060184
Jiang JX, Riquelme MA, Zhou JZ (2015) ATP, a double-edged sword in cancer. Oncoscience 2(8):673–674. https://doi.org/10.18632/oncoscience.230
Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun LZ, Jiang JX (2015) Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene 34(14):1831–1842. https://doi.org/10.1038/onc.2014.113
Liu X, Riquelme MA, Tian Y et al (2021) ATP Inhibits breast cancer migration and bone metastasis through down-regulation of CXCR4 and purinergic receptor P2Y11. Cancers (Basel) 13(17):4293. https://doi.org/10.3390/cancers13174293
Kim M, Jiang LH, Wilson HL, North RA, Surprenant A (2001) Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 20(22):6347–6358. https://doi.org/10.1093/emboj/20.22.6347
Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS (2009) Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 297(2):C430–C439. https://doi.org/10.1152/ajpcell.00079.2009
Zhang Y, Cheng H, Li W, Wu H, Yang Y (2019) Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int J Cancer 145(4):1068–1082. https://doi.org/10.1002/ijc.32207
Yuan Y, Cai X, Shen F, Ma F (2021) HPV post-infection microenvironment and cervical cancer. Cancer Lett 497:243–254. https://doi.org/10.1016/j.canlet.2020.10.034
Pfaffenzeller MS, Franciosi MLM, Cardoso AM (2020) Purinergic signaling and tumor microenvironment in cervical Cancer. Purinergic Signal 16(1):123–135. https://doi.org/10.1007/s11302-020-09693-3
Funding
This work is supported by the grants from NSFC (82074478), Jiangsu Provincial Hospital of Chinese Medicine (Y2020CX39), and Acupuncture & Chronobiology Key Laboratory of Sichuan Province (No. 2021001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Bai, C., He, XM. et al. P2X7 receptor involved in antitumor activity of atractylenolide I in human cervical cancer cells. Purinergic Signalling 19, 145–153 (2023). https://doi.org/10.1007/s11302-022-09854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09854-6