Abstract
A violacein-producing bacterium was isolated from a mud sample collected near a hot spring on Kümbet Plateau in Giresun Province and named the GK strain. According to the phylogenetic tree constructed using 16S rRNA gene sequence analysis, the GK strain was identified and named Janthinobacterium sp. GK. The crude violacein pigments were separated into three different bands on a TLC sheet. Then violacein and deoxyviolacein were purified by vacuum liquid column chromatography and identified by NMR spectroscopy. According to the inhibition studies, the HIV-1 RT inhibition rate of 1 mM violacein from the GK strain was 94.28% and the CoV-2 spike RBD:ACE2 inhibition rate of 2 mM violacein was 53%. In silico studies were conducted to investigate the possible interactions between violacein and deoxyviolacein and three reference molecules with the target proteins: angiotensin-converting enzyme 2 (ACE2), HIV-1 reverse transcriptase, and SARS-CoV-2 spike receptor binding domain. Ligand violacein binds strongly to the receptor ACE2, HIV-1 reverse transcriptase, and SARS-CoV-2 spike receptor binding domain with a binding energy of −9.94 kcal/mol, −9.32 kcal/mol, and −8.27 kcal/mol, respectively. Deoxyviolacein strongly binds to the ACE2, HIV-1 reverse transcriptase, and SARS-CoV-2 spike receptor binding domain with a binding energy of −10.38 kcal/mol, -9.50 kcal/mol, and −8.06 kcal/mol, respectively. According to these data, violacein and deoxyviolacein bind to all the receptors quite effectively. SARS-CoV-2 spike protein and HIV-1-RT inhibition studies with violacein and deoxyviolacein were performed for the first time in the literature.
Graphical abstract

Similar content being viewed by others
Introductıon
The pandemic crisis has been influencing human health globally since late 2019, because of the accelerating threat of acute respiratory syndrome CoV-2 illness caused by a novel kind of coronavirus (CoV), SARS-CoV-2 or COVID-19, first diagnosed in Huanan Seafood Wholesale Market, Wuhan City, China, which then spread throughout the world (Acter et al. 2020; Núñez-Delgado et al. 2020; Vellingri et al. 2020; Zarei et al. 2020). According to WHO data, by September 2021, worldwide there had been 230,418,451 confirmed cases of COVID-19 and 4,724,876 deaths (World Health Organization, 2021). During the SARS-CoV-2 viral infection, the receptor-binding domain (RBD) of SARS-CoV-2 spike proteins S1 subunit and S2 subunit plays an important role in the fusing of viral and host membranes (Tai et al. 2019). For virus–cell membrane fusion, the S1 subunit of SARS-CoV-2 S protein identifies and binds to cell receptor ACE2, as well as to HR1 and HR2, and this creates a six-helix bundle (Duran et al. 2021). Therefore, during the viral infection process, the RBD of SARS-CoV-2 spike protein plays a critical role in binding to the ACE2 receptor (Duran et al. 2021). HIV-1 enzymes, such as reverse transcriptase (RT), integrase (INT), and protease (PR), play a major role in the replication process. The treatment or defensive strategy for HIV-1 infection is currently through antiretrovirals targeting HIV-1 PR, INT, and RT (Yang H et al. 2012). The function of HIV-1 RT is to convert the single-stranded RNAs into double-stranded DNAs, which is vital to restraining HIV-1 replication and becomes the main target for antiviral research (Wilen et al. 2012). Therefore, the RT of HIV-1 is a significant target for the development of novel anti-HIV drugs (Yang et al. 2012; Patrikar et al. 2014; Rhee et al. 2016; Esposito et al. 2012).
Violacein and deoxyviolacein (a minor, more hydrophobic co-metabolite of violacein) are secondary metabolites produced by microorganisms for protection from environmental stress and external hazards (Pantanella et al. 2007). Violacein is produced by bacterial strains of different genera such as Janthinobacterium, Duganella, Collimonas, and Pseudoalteromonas (Baricz et al. 2018). Members of the genus Janthinobacterium are gram-negative, rod-shaped, motile, strictly aerobic, and chemoorganotrophic. Generally, they can grow at the optimum temperature of 25–30 °C (Baldani et al. 2014), although some psychrophilic isolates can grow at temperatures as low as 4 °C (Suman et al. 2015). Janthinobacterium strains inhabit a variety of aquatic habitats such as soil (Asencio et al. 2014; Shoemaker et al. 2015; Wu et al. 2017), lakes, sediments (McTaggart et al. 2015), and water cisterns (Haack et al. 2016).
Violacein is an indole derivative that occurs through the condensation of two molecules of tryptophan. It has a molecular weight of 343.33 g/mol and a clear formula, C20–H13–N3–O3 (Hoshino et al. 1987). It is needle prism-shaped, purplish-black, insoluble in water, slightly soluble in ethanol, moderately soluble in dioxane and acetone, and completely soluble in DMSO, ethyl acetate, and methanol. Its melting point is > 290 °C (decomposition) (Hoshino et al. 1987). Moreover, it shows maximum absorbance at 258, 372, and 575 nm (ε575 = 2.97 ± 0.09 × 10−2 ml/µg per cm) for ethanolic solution and 585 nm for methanolic solution in the UV/Vis spectrophotometer (Rettori and Duran 1998; Blosser and Gray 2000). The fluorescence emission spectrum at an excitation wavelength of 575 nm shows an emission band at 675 nm [1.5 µg/ml (4.4 µM)] in ethanol (Asamizu et al. 2007).
Violacein has shown antibacterial (Asencio G et al. 2014; Duran N et al. 2012; O’Sullivan et al. 1990), antileishmanial (Leon et al. 2001), antiviral (May et al. 1991; Andrighetti-Frohner 2003), antitumoral (Bromberg et al. 2010), anti-inflammatory (Duran et al. 2016), antileukemic (Ferreira et al. 2004), antimycobacterial (Duran et al. 2007), antimycotic (Duran et al. 2007), antifungal, antiparasitic, antiprotozoal, antioxidant, antinociceptive, immunomodulatory, and antiulcerogenic activity (Duran et al. 2012). Andrighetti-Frohner et al. (2003) examined the antiviral and cytotoxic activities of violacein using three different methods and they reported antiviral activity against herpes simplex virus (HSV) and poliovirus after infection of HeLa cells. Violacein did not show cytopathic or antiviral activity against HSV-1 (29-R/acyclovir resistant strain), adenovirus type 5, or hepatitis A virus (strains HN175 and HAF-203) at concentrations that did not inhibit cell growth. Via the MTT assay, slight inhibition of viral replication was observed in HSV-1 (strains KOS and ATCC/VR-733), poliovirus type 2, and simian rotavirus SA11 strains (Andrighetti-Frohner et al. 2003). May et al. (1991) suggested superior antiviral activity for the compound, with dissimilarity attributed to differences in the degrees of purity of violacein and the methods (Andrighetti-Frohner et al. 2003). The differences between these results were probably due to the use of several protocols for the purification of violacein or the degree of purity of the violacein obtained (Duran et al. 2007). Other studies have reported the cyclodextrin/violacein nanostructure (Durán et al. 1998) and the pharmacological use of cyclodextrin-Au-thiol-derivative/hydrophobic nanoparticles as an antiviral drug (Alves et al. 2005).
Due to the diversity of the biological activities of violacein, it was considered that it could be a new agent for the treatment of SARS-CoV-2 (Duran et al. 2021). Duran et al. (2021) suggest that violacein can act as a protease inhibitor at the ACE2 receptor level and so it can be used as an immunotherapeutic medicine for COVID-19. Additionally, research on HIV-1 proteins, which are responsible for reverse transcription and viral DNA integration, indicated that small molecules can have significant importance for pre-exposure HIV-1 prophylaxis (Tarasova et al. 2018).
The main objectives of the present study were to demonstrate the antiviral activity of violacein and deoxyviolacein isolated from Janthinobacterium sp. GK strain against CoV-2 spike RBD:ACE2 interaction and HIV-1-RT for the first time in the literature. Additionally, in silico studies were conducted to investigate the possible interactions between these two violacein compounds and the target proteins to support these data.
Materials and methods
Bacterial isolation and growth conditions
A violacein-producing bacterium was isolated from a mud sample collected near a hot spring on Kümbet Plateau in Giresun Province and named the GK strain. This bacterium was grown on nutrient agar medium at 20–25 °C for 48 h.
Identification of the bacterial strain using 16S rRNA gene sequencing
Bacterial genomic DNA was first isolated and the 16S rRNA gene was amplified from the genomic DNA using oligonucleotide primers designed for conserved positions in the 3′ and 5′ regions of bacterial 16S rRNA genes. UNI16S-L (5’-ATTCTAGAGTT TGATCATGGCTTCA) was used as forward primer and UNI16S-R (5’-ATGGTACCGTGTGACGGGCGGTGTTGTA) as reverse primer for amplification. The PCR reaction conditions used were those reported by Beff et al. (1996). The PCR fragments were cloned into a pGEM-T vector system I and sequenced by Macrogen Europe (Amsterdam, the Netherlands).
16S rRNA gene sequence similarities were calculated using the EzBioCloud Database (https://www.ezbiocloud.net/; Yoon et al. 2017). Multiple alignments of sequences from closely related strains were performed using the ClustalW in MEGA version X software (Kumar et al. 2018). By using related sequences, a phylogenetic tree was reconstructed via the neighbor-joining method (Saitou and Nei 1987).
Violacein pigment extraction from the GK strain
The GK strain was grown on nutrient agar medium at 20 °C for up to 6 days. The pellet obtained from the bacteria grown was washed with dH2O. Then a 100-mg pellet was dissolved in 1 ml of methanol. The dissolved pellet was incubated in a shaker at room temperature for 1 h and then centrifuged for 10 min at 10,000 rpm and the supernatant was removed. The processes were repeated from the solvent addition step until the pellet turned from purple to gray-white. The supernatants collected were evaporated with an evaporator, leaving some solvents. It was kept at 40 °C overnight to evaporate the remaining solvent (Yada et al. 2008).
Thin layer chromatography (TLC)
Determination of the violacein pigment was carried out on TLC pre-coated silica gel aluminum sheets (Merck Silica gel 60 F254) using a mobile phase containing isopropanol/ammonia/water in the ratio 8:1:1 (v/v) (Malar et al. 2014). Next, 4, 3, 2, and 1 mg/mL crude violacein extracts in 10 µL aliquots were applied slowly by micropipette onto the silica sheet. The samples were allowed to run for about three-fourths of the plate. After development, the plate was dried and the bands were visualized by UV irradiation. The following formula was used for the calculation of the retention factor (Rf):
Purification of violacein extract
The violacein pigment was first extracted with ethanolic solution, then filtered, and evaporated under reduced pressure at 50 °C, yielding 590 mg of crude extract. Then this mass underwent continuous Soxhlet extraction, first with chloroform, second with diethyl ether (for 3 ± 4 h), and third with ethanol for 3 h each (Rettori and Duran 1998). Evaporation of the chloroform, ether, and ethanol extracts under reduced pressure gave 197 mg, 8.4 mg, and 90.4 mg of semipurified extracts, respectively. The crude ethanolic violacein extract (90.4 mg) was dissolved in MeOH (30 mL) and impregnated with silica (15 g); then the solvent was evaporated. A sample impregnated with dry silica was loaded on a column, which was subjected to vacuum liquid column chromatography (VLCC, Silica gel 60, 230–400 mesh) by gradient elution of n-hexane (100 mL), n-hexane/chloroform (30:10, 30:20, 100 mL each), chloroform (100 mL), chloroform/methanol (50:3, 50:4, 60:4, 60:5, 40:20, 50:50, 100 mL each), and methanol (100 mL) solvent and solvent mixtures to give 32 subfractions (~ 30 mL, each). The fractions were analyzed by proton (300 MHz) and carbon-13 (75 MHz) 1H-NMR spectroscopy (Rettori and Duran 1998).
Antiviral assays
HIV-1 reverse transcriptase inhibition assay
The isolated and purified violacein and deoxyviolacein pigments were assayed for their inhibitory activity against recombinant HIV-1 RT in vitro using a non-radioactive HIV-1 RT colorimetric ELISA kit from Roche Diagnostics (Germany). The protocol outlined in the kit was followed using 4 ng of HIV-1 RT in a well and incubating the reaction mixture containing 1, 0.5, 0.3, 0.2, 0.1, and 0.05 mM violacein and 1.5, 1, 0.75, and 0.5 mM deoxyviolacein pigments for 1 h at 37 °C. Nevirapine was used as a positive control. The absorbance of the samples was measured at 405 nm (reference wavelength: approx. 490 nm) using a UV/Vis spectrophotometer microplate (ELISA) reader. The assay was carried out in triplicate.
Inhibition assay for COVID-19
For screening inhibitors of the CoV-2 spike RBD:ACE2 interaction, the Spike S1 (SARS-CoV-2): ACE2 inhibitor scanning colorimetric assay kit (Cat. No. 79954, BPS Bioscience, San Diego, CA, USA) was used. Based on the protocol of the kit, 10 µl of test substances containing 2, 1.5, 1, 0.75, 0.5, 0.25, and 0.1 mM violacein and 1, 0.75 and 0.5 mM deoxyviolacein were placed in wells followed by incubation for 1 h at room temperature with slow shaking. Absorbance was measured at 450 nm using UV/Vis spectrophotometer microplate reader at the end of the COVID-19/ELISA test procedure. All tests were performed in triplicate.
Molecular docking studies
In silico studies were conducted using AutoDock 4.2 software to investigate the possible interactions between violacein and three reference molecules and the target proteins (Morris et al. 2009). Three proteins were used as the target: angiotensin-converting enzyme 2 (ACE2), HIV-1 reverse transcriptase, and SARS-CoV-2 spike receptor binding domain. The crystal structures for those proteins were downloaded from the RCSB Protein Data Bank (http://www.rcsb.org/pdb) with the following IDs: 6M0J for the ACE2 (2.45 Å), 3T19 for the HIV-1 reverse transcriptase (2.60 Å), and 6YLA for the SARS-CoV-2 spike receptor binding domain (2.42 Å). The ligand structure was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) in.sdf format and converted into.pdb files using OpenBabelGUI 2.4.1 software (O'Boyle et al. 2011). Before docking, each ligand was optimized in terms of torsional barriers, intermolecular-interaction geometry, and molecular geometry using the universal force field with Avogadro software (Hanwell et al. 2012). All nonstandard residues, water molecules, and other ligands were removed from the receptors, and then polar hydrogens and Gasteiger charges were added to the receptors files, consecutively, which were then converted to PDBQT files used for docking by the program MGL Tools. The prepared ligand and proteins were used as input files for AutoDock 4.2 software (Morris et al. 2009) and the Lamarckian genetic algorithm was employed for all docking simulations. All dockings experiments were performed as blind docking to ascertain the probable binding sites. Further, the entire receptor molecules were covered with a grid box of dimension 126 Å × 126 Å × 126 Å with grid spacing of 0.375 Å. The receptor proteins were kept rigid while the ligand was kept flexible. The docking studies were performed with 150 individuals in the population, maximum generation of 54,000, and maximum energy evaluations of 2,500,000 to result in 100 genetic algorithm runs. The default settings were applied for all other parameters. The binding free energies of the docked conformations of the ligand against the receptor proteins were analyzed using MGL Tools 1.5.6 (Morris et al. 2009). The docking results described the affinity represented by the docking score and binding interaction (hydrogen/hydrophobic) of the ligand on the protein of interest. The binding result table and the best model for each receptor–ligand interaction were saved for visualization. The hydrophilic and hydrophobic interactions were determined and post-docking analysis of protein–ligand interactions was conducted using the BIOVIA Discovery Studio Visualizer (Dassault Systèmes BIOVIA, 2016).
Results
Bacterial identification
Depending on the 16S rRNA gene (1354 bp) analysis results, the GK strain showed 99.78% similarity to Janthinobacterium lividium (Y08846). The phylogenetic tree was constructed using 16S rRNA gene sequence information from the strains shown in Fig. 1. According to this phylogenetic tree, the isolated GK strain was identified as Janthinobacterium sp. strain GK. The 16S rRNA gene sequence of Janthinobacterium sp. GK has been deposited in the GenBank database with the accession number MZ434955.
The phylogenetic tree constructed using sequence information of 16SrRNA gene from Jantinobacterium sp. GK. The neighbour-joining methods with bootstrap tests of 1000 replicants were performed using MEGA 7.0. Pelomonas saccharophilia DSM 654 used as an outgroup (GenBank accession number: MZ434950, MZ434955)
TLC and purification
The crude violacein pigment was separated into three different bands on a TLC sheet. The distance traveled by the solvent was 12.6 cm and the distances traveled by the bands of crude violacein of the GK strain were 1.2 cm, 8.2 cm, and 9.7 cm, respectively. The Rf values of the GK strain bands were calculated as 0.095, 0.65, and 0.77 cm, respectively (Fig. 2).
Violacein and deoxyviolacein were purified by VLCC and gave 32 subfractions. After the TLC control and 1H-NMR spectroscopy, subfractions 3–5 (deoxyviolacein, 18.3 mg), 18–21 (mixture, 25.9 mg), and 22–29 yielded violacein (45.3 mg). Subfractions that were identified as violacein and deoxyviolacein were combined separately and used for inhibition tests.
Antiviral assays
HIV-1 reverse transcriptase inhibition assay
According to the inhibition test results, when 1 mM, 0.5 mM, 0.3 mM, 0.2 mM, 0.1 mM, and 0.05 mM violacein from the GK strain were used, the inhibition rates were 97.14%, 94.28%, 87.98%, 61.95%, 32.08%, 27.87%, and 8.75%, respectively (Fig. 3a). When 1.5 mM, 1 mM, 0.75 mM, and 0.5 mM concentrations of deoxyviolacein from the GK strain were used, the inhibition rates were 20.5%, 17.68%, 14.37%, and 12.78%, respectively (Fig. 3a).
Inhibition assay for COVID-19
When 2 mM, 1.5 mM, 1 nM, 0.75 mM, 0.5 mM, 0.25 mM, and 0.1 mM concentrations of violacein from the GK strain were used, the inhibition rates were 53%, 44%, 31.5%, 23%, 18%, 15%, and 1%, respectively (Fig. 3b). When 1 mM, 0.75 mM, and 0.5 mM concentrations of deoxyviolacein from the GK strain were used, the inhibition rates were 14.5%, 7.55%, and 5.5%, respectively (Fig. 3b).
In silico studies
Significant interactions between the ligands and the target protein were obtained by successful docking of violacein, deoxyviolacein, and the reference molecule (Table 1) with ACE2. Ligand violacein strongly binds to the receptor with a lower binding energy (−9.94 kcal/mol) and Ki value (51.66 nM), while the reference molecule (hydroxychloroquine) has a binding energy of −7.90 kcal/mol and Ki value of 1.61 µM. Violacein formed 3 conventional hydrogen bonds with the receptor protein, and their atomic distances were lower than 3 Å. These are Glu435, Ile291, and His540 residues with the lengths of 2.99 Å, 2.33 Å, and 1.91 Å, respectively. In addition, violacein formed a pi–lone pair bond at the Asn290 position 2.91 Å in length (Fig. 4). Violacein strongly binds to the HIV-1 reverse transcriptase receptor with a lower binding energy (−9.32 kcal/mol) and Ki value (146.32 nM), while the reference molecule (nevirapine) has a binding energy of −9.35 kcal/mol and Ki value of 140.44 µM. The ligand formed two pi–pi stacked, one pi–pi T-shaped, and eleven pi–alkyl bonds with the receptor protein. Two of these bonds have an atomic distance of less than 4 Å and only one of them has an atomic distance of less than 3 Å (Fig. 5). When the docking results with the SARS-CoV-2 spike receptor binding domain, which is the third receptor, are examined, it is seen that violacein binds much better with a lower binding energy (−8.27 kcal/mol) and Ki value (866.65 nM) than the reference molecule hydroxychloroquine (binding energy: −6.32 kcal/mol and Ki value 23.35 µM). When the bonds are examined, it is seen that five conventional hydrogen bonds are formed, four of which are below 3 Å and two of them are stronger below 2 Å at positions Asp428 and Thr430 with lengths of 1.92 Å and 1.66 Å, respectively (Fig. 6).
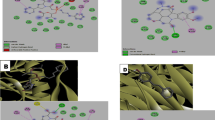
Binding pose profile of (1) violacein and (2) deoxyviolacein in the target protein HIV-1 reverse transcriptase a, red shaped molecule represents the receptor, and yellow shaped molecule indicates the ligand. 2D b and 3D c representations of HIV-1 reverse transcriptase with violacein and deoxyviolacein
Binding pose profile of (1) violacein and (2) deoxyviolacein in the target protein SARS-CoV-2 Spike receptor-binding domain a, red shaped molecule represents the receptor, and yellow shaped molecule indicates the ligand. 2D b and 3D c representations of SARS-CoV-2 Spike receptor binding domain with violacein and deoxyviolacein
After the successful docking of deoxyviolacein (Table 1), the results showed us significant interactions between the ligand and the three target proteins, similar to violacein. Deoxyviolacein strongly binds to the ACE2 with a binding energy of −10.38 kcal/mol and Ki value of 24.58 nM, HIV-1 reverse transcriptase with a binding energy of −9.50 kcal/mol and Ki value of 108.13 nM, and SARS-CoV-2 spike receptor binding domain with a binding energy of −8.06 kcal/mol and Ki value of 1.23 µM, while the reference molecule (hydroxychloroquine) has a higher binding energy (−7.90 kcal/mol) and Ki value (1.61 µM) for ACE2 and for SARS-CoV-2 spike receptor binding domain (binding energy −6.32 kcal/mol and Ki value 23.35 µM).
Discussion
In the present study, the 16S rRNA gene from the isolated GK strain was cloned into a pGEM-T vector system I, sequenced, and identified as Janthinobacterium sp. GK. The GK strain showed 99.78% similarity to Janthinobacterium lividium (Y08846), 99.56% similarity to Janthinobacterium rivuli (MN548379), and 99.48% similarity to Janthinobacterium aquaticum (MN548378). The results of the phylogenetic analysis supported the affiliation of the GK strain to the genus Janthinobacterium. It was also determined that the GK strain clusters together with Janthinobacterium lividium in the phylogenetic tree, which is the closest species according to the 16S rRNA gene similarity results.
According to a study performed by Malar et al. (2014), as a result of mobile phase TLC with violacein pigment dissolved in methanol obtained from Chromobacterium sp. violacein extract, the Rf value was 0.6. In our study, for screening violacein the same protocol was applied for crude violacein extract of Jantinobacterium sp. GK strain and the Rf value of the second band visualized in TLC was 0.65, which was very similar to that of the violacein of Chromobacterium sp.
Violacein and deoxyviolacein were purified by VLCC and the fractions identified were combined separately and used for inhibition tests. The aim of the SARS-CoV-2 Spike S1:ACE2 biotin binding inhibition test is to prevent the virus from entering the cell by preventing the interaction between CoV-2 spike RBD and ACE2 and showing the effect of violacein on the interaction between CoV-2 spike RBD and ACE2. The results showed that CoV-2 spike RBD:ACE2 inhibition depends on the concentration of the violacein and deoxyviolacein (dissolved with 10% DMSO). The highest concentration of violacein (2 mM) showed 53% inhibition. When we compared the inhibition rates of violacein and deoxyviolacein on SARS-CoV-2 spike S1:ACE2 biotin binding at the same concentrations (1 mM, 0.75 mM, and 0.5 mM) all concentrations of violacein were more effective on SARS-CoV-2 than deoxyviolacein. Additionally, the lowest concentration of violacein (0.25 mM) exhibited almost the same inhibition rate as the highest concentration of deoxyviolacein (1 mM) for SARS-CoV-2. The HIV-1 RT inhibition test results showed that violacein at the same concentration (0.5 mM) provided greater inhibition than deoxyviolacein. In addition, the second-lowest concentration of violacein (0.1 mM) exhibited almost the same rate of inhibition as the highest concentration of deoxyviolacein (1 mM) for HIV-1 RT. As a result, violacein showed more efficient inhibition and deoxyviolacein showed lower inhibitory activity.
In the literature, the antiviral action of violacein from C. violaceum (0.078 to 2.5 µM) against HSV-1 (strains KOS, ATCC/VR733, and 29-R/acyclovir resistant), RV-SA11, PV-2, HAV (strains HM175 and HAF-203), and AdV-5 were studied with the MTT assay and cytopathogenicity inhibition test (Andrighetti-Frohner et al., 2003). As a result, violacein indicated no cytopathic effect inhibition on HSV-1 (strain 29-R/acyclovir resistant), HAV (strains HM175 and HAF-203), or AdV-5. None of them showed antiviral activity according to the MTT assay. The concentrations of violacein indicated weak inhibition of HSV-1 (strains KOS and ATCC/VR733), PV-2, and RV-SA11 replication through the MTT assay. The percentages of viral inhibition were less than 50% between 0.312 and 1.25 µM concentrations. Violacein (with 10% of deoxyviolacein) showed excellent virustatic activity against herpes simplex viruses and polioviruses type 1 cure and prophylaxis according to May et al. (1991). A concentration of 0.198 μg/ml violacein results in cell protection of 50%. Because of the use of different protocols for violacein extraction by the two research groups, the extracts may include different ingredients. Rettori and Duran (1998) had a patent for a cyclodextrin/violacein mixture for treating viral, bacterial, trypanocidal infections and for antitumoral activity. Duran and Menck (2001) indicated that 0.063 µg/ml violacein inhibited poliovirus replication at the rate of 56% and 0.25 µg/ml violacein inhibited HSV replication at the rate of 62% in HeLa cells. However, since this information is obtained from patent documents, there are no details about the virus strains or types, or the experimental conditions under which the results were obtained.
HIV-RT colorimetric ELISA kit was used in a previous study by Sanna et al. (2020) to evaluate two ethyl acetate extracts from Thymelae hirsuta, first from leaves (1 B) and second from branches (72 B) for anti-HIV type 1 activity. Their results showed that the branches extract (72 B) exhibits potent and selective activity against HIV-1 wt (EC50 = 0.8 μg/mL) at noncytotoxic concentrations (CC50 > 100 μg/mL). A CoV-2 spike RBD:ACE2 inhibitor scanning colorimetric assay kit (Cat. No. 79954) was also used previously for screening and profiling inhibitors of the RBD/ACE2 interaction. For example, Yang et al. (2021) used corilagin for testing, while Carino et al. (2020) used natural and semisynthetic agents.
There are many computer-based drug design methods such as molecular docking and molecular dynamics simulations to investigate possible medications. Molecular docking is mostly used to investigate various types of binding interactions of potential drugs with different sites or active sites on target molecules. Among all the different types of interaction such as H-bond, π–π, and amide–π interactions, the binding efficiency of a ligand molecule with a target has been extensively explained by evaluating the hydrogen bonding pattern and the nature of residues present in the active site. Binding free energy (kcal/mol) allows us to examine and compare the binding affinity of different ligands with their respective target receptors molecules. A lower binding energy indicates a higher affinity of the ligand for the receptor (Raj et al. 2019; Chen et al. 2019). For the present study, violacein and deoxyviolacein with broad ranges of biological activity were used to determine possible interactions against the target proteins by in silico analysis. Molecular docking studies were performed for the evaluation of the inhibitory nature of the ligands examined against three proteins. These simulations gave the predicted protein–ligand binding energies and Ki values and identified the potential ligand binding sites. When the docking results of violacein with the HIV-1 reverse transcriptase were examined, it was seen that the ligand binds to the reference molecule nevirapine at a similar level. Violacein and deoxyviolacein appear to bind better than the reference molecule hydroxychloroquine to ACE2 and SARS-CoV-2 spike receptor binding domain. When all docking results were evaluated together, it was seen that violacein and deoxyviolacein bind to all the receptors quite effectively. However, when the binding energies are considered, deoxyviolacein binds to ACE2 and HIV-1 reverse transcriptase slightly better than violacein does. However, when we consider in vitro studies, it was found that violacein has much better inhibition rates than deoxyviolacein. In the literature, in vitro and in silico studies do not always agree with each other (Rotich et al. 2021).
In conclusion, the effects of violacein and deoxyviolacein isolated from Jantinobacterium sp. GK strain on SARS-CoV-2 spike S1:ACE2 biotin binding, and HIV-1 RT inhibition were shown for the first time herein. Our study is an example showing that in vitro and in silico studies are not always compatible. Therefore, in silico studies should always be supported by in vitro studies. Finally, according to the in vitro and in silico studies, especially because of violacein’s inhibitory effects, it has great potential as a drug candidate.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Acter T, Uddin N, Das J, Akhter A, Choudhury TR, Kim S (2020) Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci Total Environ 730:138996. https://doi.org/10.1016/j.scitotenv.2020.138996
Almerico AM, Tutone M, Lauria A (2008) Docking and multivariate methods to explore HIV-1 drug-resistance: a comparative analysis. J Comput Aid Mol Des 22:287–297. https://doi.org/10.1007/s10822-008-9186-7
Alves OL, Gimenez IF, De Azevedo MMM, Durán N, Melo PS (2005) Pharmacological use of cyclodextrineAu-thiol-derivative/hydrophobic compound nanoparticles as antitumoral, antibacterial, antiviral and/or antiparasites, its obtention process and formulation. Brazil Pat; PIBr 0502657-1
Andrighetti-Frohner CR, Antonio RV, Creczynski-Pasa TB, Barandi CRM, Simoes CMO (2003) Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz 98:834–848. https://doi.org/10.1590/S0074-02762003000600023
Asamizu S, Kato Y, Yasuhiro I, Onaka H (2007) VioE, a prodeoxyviolacein synthase involved in violacein biosynthesis, it responsible for intramolecular indole rearrangement. Tetrahedron Lett 48:2923–2926. https://doi.org/10.1016/J.TETLET.2007.02.062
Asencio G, Lavin P, Alegría K, Domínguez M, Bello H, González-Rocha G, González-Aravena M (2014) Antibacterial activity of the Antarctic bacterium Janthinobacterium sp. SMN 33.6 against multi-resistant gram-negative bacteria. Electron J Biotech 17(1):1–5. https://doi.org/10.1016/j.ejbt.2013.12.001
Baldani IJ, Rouws L, Cruz LM, Olivares FL, Schmid M, Hartmann A (2014) The family Oxalobacteraceae. In: Rosenverg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes—alphaproteobacteria and betaproteobacteria. Springer-Verlag, Berlin/Heidelberg, pp 919–974
Baricz A, Teban A, Chiriac CM, Szekeres E, Farkas A, Nica M, Dascalu A, Oprisan C, Lavin P, Coman C (2018) Investigating the potential use of an Antarctic variant of Janthinobacterium lividum for tackling antimicrobial resistance in a one health approach. Sci Rep 8:15272. https://doi.org/10.1038/s41598-018-33691-6
Beffa T, Blanc M, Lyon PF, Vogt G, Marchiani M, Fischer JL, Aragno M (1996) Isolation of Thermus strains from hot composts (60 to 80 °C). Appl Environ Microbiol 62:1723–1727. https://doi.org/10.1128/aem.62.5.1723-1727.1996
Blosser RS, Gray KM (2000) Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassey for N-acyl homoserine lactone autoinducers. J Microbiol Methods 40:47–55. https://doi.org/10.1016/s0167-7012(99)00136-0
Bromberg N, Dreyfuss JL, Regatieri CV, Palladino MV, Durán N, Nader HB, Haun M, Justo GZ (2010) Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem Biol Interact 186(1):43–52. https://doi.org/10.1016/j.cbi.2010.04.016
Carino A, Moraca F, Fiorillo B, Marchianò S, Sepe V, Biagioli M, Finamore C, Bozza S, Francisci D, Distrutti E, Catalanotti B, Zampella A, Fiorucci S (2020) Hijacking SARS-CoV-2/ACE2 receptor interaction by natural and semi-synthetic steroidal agents acting on functional pockets on the receptor binding domain. Front Chem 23(8):572885. https://doi.org/10.3389/fchem.2020.572885
Checkley MA, Luttge BG, Freed EO (2011) HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol 410(4):582–608. https://doi.org/10.1016/j.jmb.2011.04.042
Chen D, Oezguen N, Urvil P, Ferguson C, Dann SM, Savidge TC (2016) Regulation of protein–ligand binding affinity by hydrogen bond pairing. Sci Adv 2(3):e1501240. https://doi.org/10.1126/sciadv.1501240
Dassault Systèmes BIOVIA (2016) Discovery Studio Modeling Environment, Release 2017. Dassault Systèmes, San Diego
Durán N, Justo GZ, Ferreira CV, Melo PS, Cordi L, Martins D (2007) Violacein: properties and biological activities. Biotechnol Appl Biochem 48:127–133. https://doi.org/10.1042/BA20070115
Durán N, Justo GZ, Durán M, Brocchi M, Cordi L, Tasic L, Castro GR, Nakazato G (2016) Biotechnol Adv 34(5):1030–1045. https://doi.org/10.1016/j.biotechadv.2016.06.003
Duran M, Ponezi AN, Faljoni-Alario A, Teixeira MFS, Justo GZ, Duran N (2012) Potential applications of violacein: a microbial pigment. Med Chem Res 21:1524–1532. https://doi.org/10.1007/s00044-011-9654-9
Duran N, Justo GZ, Nakazato G, Fávaro WJ (2021) Violacein, a microbial antiviral product: does play key role as active agent against SARS-CoV-2? AIJR Preprints. https://doi.org/10.21467/preprints.315
Esposito F, Corona A, Tramontano E (2012) HIV-1 reverse transcriptase still remains a new drug target: structure, function, classical inhibitors, and new inhibitors with innovative mechanisms of actions. Mol Biol Int 2012:586401. https://doi.org/10.1155/2012/586401
Ferreira CV, Bos CL, Versteeg HH, Justo GZ, Durán N, Peppelenbosch MP (2004) Molecular mechanism of violacein-mediated human leukemia cell death. Blood 104(5):1459–1464. https://doi.org/10.1182/blood-2004-02-0594
Haack FS, Poehlein A, Kröger C, Voigt CA, Piepenbring M, Bode HB, Daniel R, Schäfer W, Streit WR (2016) Molecular keys to the Janthinobacterium and Duganella spp. interaction with the plant pathogen Fusarium graminearum. Front Microbiol 7:1668. https://doi.org/10.3389/fmicb.2016.01668
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17. https://doi.org/10.1186/1758-2946-4-17
Hoshino T, Kondo T, Uchiyama T, Ogasawara N (1987) Studies on the biosynthesis of violacein. 1. biosynthesis of violacein - a novel rearrangement in tryptophan-metabolism with a 1,2-shift of the indole ring. Agr Biol Chem Tokyo 51:965–968. https://doi.org/10.1080/00021369.1987.10868084
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Leon LL, Miranda CC, De Souza AO (2001) Durán N (2001) Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J Antimicrob Chemo 48(3):449–450. https://doi.org/10.1093/jac/48.3.449
Malar SEE, Krishnaveni N, Jayakrishnan A, Ronald, (2014) Extraction and characterization of the pigment violacein from Chromobacterium violaceum and ıts antibacterial properties. PSGCAS Search: J Sci Technol 22(30–5):2349–5456
McTaggart TL, Shapiro N, Woyke T, Chistoserdova L (2015) Draft genome of Janthinobacterium sp. RA13 isolated from Lake Washington sediment. Genome Annouc 3:13–14. https://doi.org/10.1128/genomeA.01588-14
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Nu´nez-Delgado A, (2020) What do we know about the SARS-CoV-2 coronavirus in the environment? Sci Total Environ 727:138647. https://doi.org/10.1016/j.scitotenv.2020.138647
O’sullivan J, McCullough J, Johnson JH, Bonner DP, Clark JC, Dean L, Trejo WH (1990) Janthinocins A, B and C, novel peptide lactone antibiotics produced by Janthinobacterium lividum. I. Taxonomy, fermentation, isolation, physico-chemical and biological characterization. J Antibiot 43:913–919. https://doi.org/10.7164/antibiotics.43.913
O’Boyle M, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open babel: an open chemical toolbox. J Cheminf 3:33. https://doi.org/10.1186/1758-2946-3-33
Pantanella F, Berlutti F, Passariello C, Sarli S, Morea C, Schippa S (2007) Violacein and biofilm production in Janthinobacterium lividum. J Appl Microbiol 102:992–999. https://doi.org/10.1111/j.1365-2672.2006.03155.x
Patrikar S, Basannar DR, Bhatti VK, Kotwal A, Gupta RM, Grewal RS (2014) Rate of decline in CD4 count in HIV patients not on antiretroviral therapy. Med J Armed Forces India 70(2):134–138. https://doi.org/10.1016/j.mjafi.2013.08.005
Raj S, Sasidharan S, Dubey VK, Saudagar P (2019) Identification of lead molecules against potential drug target protein MAPK4 from donovani: an in-silico approach using docking, molecular dynamics and binding free energy calculation. PLoS ONE 14(8):e0221331. https://doi.org/10.1371/journal.pone.0221331
Rettori D, Duran N (1998) Production, extraction and purification of violacein: an antibiotic pigment produced by Chromobacterium violaceum. World J Microbiol Biotechnol 14:685–688. https://doi.org/10.1023/A:1008809504504
Rhee SY, Sankaran K, Varghese V, Winters MA, Hurt CB, Eron JJ, Parkin N, Holmes SP, Holodniy M, Shafer RW (2016) HIV-1 protease, reverse transcriptase, and integrase variation. J Virol 90(13):6058–6070. https://doi.org/10.1128/JVI.00495-16
Rotich W, Sadgrove NJ, Mas-Claret E, Padilla-González GF, Guantai A, Langat MK (2021) HIV-1 reverse transcriptase ınhibition by major compounds in a kenyan multi-herbal composition (CareVid™): In Vitro and In Silico Contrast. Pharmaceuticals 14(10):1009. https://doi.org/10.3390/ph14101009
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Sanna G, Madeddu S, Murgia G, Serreli G, Begala M, Caboni P, Incani A, Franci G, Galdiero M, Giliberti G (2020) Potent and selective activity against human immunodeficiency virus 1 (HIV-1) of Thymelaea hirsuta extracts. Viruses 12(6):664. https://doi.org/10.3390/v12060664
Shoemaker WR, Muscarella ME, Lennon JT (2015) Genome sequence of the soil bacterium Janthinobacterium sp. KBS0711. Genome Annouc 3(3):e00689-e715. https://doi.org/10.1128/genomeA.00689-15
Suman R, Sharma P, Gupta S, Sourirajan A, Dev K (2015) A novel psychrophilic Janthinobacterium lividum MMPP4 isolated from Manimahesh Lake of Chamba District of Himachal Pradesh. India J Biochem Technol 6:846–851
Tai W, He L, Pu ZX, J, Voronin D, Jiang S, Zhou Y, Du L, (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17:613–620. https://doi.org/10.1038/s41423-020-0400-4
Tarasova O, Poroikov V, Veselovsky A (2018) Molecular docking studies of HIV-1 resistance to reverse transcriptase inhibitors: mini-review. Molecules 23:1233. https://doi.org/10.3390/molecules23051233
Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, Giridharan B, Ganesan S, Venugopal A, Venkatesan D, Ganesan H, Rajagopalan K, Rahman PKSM, Cho S, Kumar NS, Subramaniam MD (2020) COVID-19: a promising cure for the global panic. Sci Total Environ 725:138277. https://doi.org/10.1016/j.scitotenv.2020.138277
Wilen CB, Tilton JC, Doms RW (2012) HIV: Cell binding and entry. Cold Spring Harb Perspect Med 2(8):a006866. https://doi.org/10.1101/cshperspect.a006866
World Health Organisation (2021, September 24). WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
Wu X, Deutschbauer AM, Kazakov AE, Wetmore KM, Cwick BA, Walker RM, Novichkov PS, Arkin AP, Chakraborty R (2017) Draft genome sequences of two isolated from pristine groundwater collected from the Oak Ridge field research center. Genome Annouc 5:e00582. https://doi.org/10.1128/genomeA.00582-17
Yada S, Wang Y, Zou Y, Nagasaki K, Hosokawa K, Osaka I, Arakawa R, Enomoto K (2008) Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar Biotechnol 10:128–132. https://doi.org/10.1007/s10126-007-9046-9
Yang H, Nkeze J, Zhao RY (2012) Effects of HIV-1 protease on cellular functions and their potential applications in antiretroviral therapy. Cell Biosci 2(1):32. https://doi.org/10.1186/2045-3701-2-32
Yang LJ, Chen RH, Hamdoun S, Coghi P, Ng JPL, Zhang DW, Guo X, Xia C, Law BYK (2021) Wong VKW (2021) corilagin prevents SARS-CoV-2 infection by targeting RBD-ACE2 binding. Phytomedicine 87:153591. https://doi.org/10.1016/j.phymed.2021.153591
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617
Zarei A, Fardood ST, Moradnia F, Ramazani A (2020) A review on coronavirus family persistency and considerations of novel type, COVID-19 features. Eurasian Chem Commun 2:798–811. https://doi.org/10.33945/SAMI/ECC.2020.7.7
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MAD, FAS HI G, HK, EC, GB, NY. The first draft of the manuscript was written by MAD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of ınterest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
No ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogancı, M.A., Ay Sal, F., Guler, H.I. et al. Investigation of potential inhibitor properties of violacein against HIV-1 RT and CoV-2 Spike RBD:ACE-2. World J Microbiol Biotechnol 38, 161 (2022). https://doi.org/10.1007/s11274-022-03350-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03350-0