Abstract
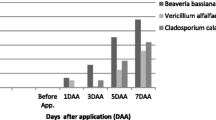
Given the aphids high reproductive capacity, assessing their biocontrol by using entomopathogenic fungi is crucial; to determine their potential, fungi were tested in planta and in field conditions. Significant decrease of Myzus persicae (Sulzer) population was observed in planta after applying Beauveria bassiana (strain 7R), Trichoderma gamsii (strain Z) or Metarhizium brunneum (strain Meta Br1) at 1 × 107 or 1 × 108 conidia/mL on pepper plants. Significant differences of aphids’ populations were detected between fungus concentration and control (F = 68.743, df = 6.980, P < 0.001), where M. brunneum at 1 × 108 conidia/mL reduced aphids population close to zero. At 20 °C, dead aphids’ mycosis by B. bassiana and T. gamsii was 78% and 84%; at 25 °C was 83% and 88%; and at 30 °C was 75% and 79%, respectively. In field conditions, Mexican PTG4 and commercial GHA B. bassiana strains were tested [(1 × 106 conidia/mL + corn starch) seed treatments] against the Melanaphis sacchari (Zehntner) aphid populations, on naturally infested sorghum plants. Results showed that plant germination and emergence were not affected, whereas yield (grams of sugar/plant) was significantly higher among treated compared with untreated plants. The aphid population decreased in plants from PTG4 treated seeds; indeed, this treatment had a significant positive effect on the flowering index, whereas the stem fresh weight and juice volume was significantly increased among plants from GHA treated seeds. Taken together, tested strains can be used as a tool to control aphids’ population on several crops such as pepper and even increase the yield in sorghum.




Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are shown within the text, tables and figures. Any further inquiry would be sent to the first and the corresponding author.
References
Battaglia D, Bossi S, Cascone P, Digilio MC, Duran Prieto J, Fanti P, Guerrieri E, Iodice L, Lingua G, Lorito M, Maffei ME, Massa N, Ruocco M, Sasso R, Trotta V (2013) Tomato Below Ground–Above Ground Interactions: Trichoderma longibrachiatum Affects the Performance of Macrosiphum euphorbiae and Its Natural Antagonists. MPMI 26:1249–1256. https://doi.org/10.1094/MPMI-02-13-0059-R
Bauer M (2015) https://flacrops.com/2015/07/17/sugarcane-aphids-building/
Baverstock J, Roy HE, Clark SJ, Alderson PG, Pell JK (2006) Effect of fungal infection on the reproductive potential of aphids and their progeny. J Invert Path 91:136–139
Bowling R, Brewer M, Knutson A, Way M, Porter P, Bynum E, Allen C, Villanueva R (2015) Scouting Sugarcane Aphids http://agrilife.org/ccag/files/2015/05/ScoutCard.pdf
Bowling RD, Brewer MJ, Kerns DL, Gordy J, Seiter N, Elliott NE, Buntin GD, Way MO, Royer TA, Biles S, Maxson E (2016) Sugarcane aphid (Hemiptera: Aphididae): a new pest on sorghum in North America. J Integr Pest Manag 7(1):12. doi: https://doi.org/10.1093/jipm/pmw011
Coppola M, Cascone P, Lelio ID, Woo SL, Lorito M, Rao R, Pennacchio F, Guerrieri E, Digilio MC (2019) Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front Physiol 10:813. https://doi.org/10.3389/fphys.2019.00813
De Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Contr 43:237e256
Dorschner KW, Feng MG, Baird CR (1991) Virulence of an aphid-derived isolate of Beauveria bassiana (Fungi: Hyphomycetes) to the hop aphid, Phorodon humuli (Homoptera: Aphididae). Environ Entomol 20:690–693
Ekesi S, Akpa AD, Onu I, Ogunlana MO (2000) Entomopathogenicity of Beauveria bassiana and Metarhizium anisopliae to the cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae). Arch. Phytopathol. Plant Prot 33:171–180
Fan Y, Fang W, Guo S, Pei X, Zhang Y, Xiao Y, Li D, Jin K, Bidochka MJ, Pei Y (2007) Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl Environ Microbiol 73:295–302. https://doi.org/10.1128/AEM.0197406
Filho MM, Oliveira SOD, De Liz RS, Faria M (2011) Cage and field assessments of Beauveria bassiana-based mycoinsecticides for Myzus persicae Sulzer (Hemiptera: Aphididae) control in cabbage. Neotrop Entomol 40:470–476
Gurulingappa P, McGee PA, Sword G (2011) Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop Prot 30:349–353
Hall RE, Burges HD (1979) Control of aphids in glasshouses with the fungus, Verticillium lecanii. Ann Appl Biol 93:235–246
He H-G, Li Z-Y (2008) Effects of Beauveria bassiana on the fecundity and population parameters of Myzus persicae at different temperatures. Chin Bull Entomol 45:101–104
Hesketh H, Alderson PG, Pye BJ, Pell JK (2008) The development and multiple uses of a standardized bioassay method to select hypocrealean fungi for biological control of aphids. Biol Control 46(2):242–255
Jandricic S, Filotas M, Sanderson J, Wraight S (2014) Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol., 118:34–46
Kim JJ, Kim KC (2008) Selection of a highly virulent isolate of Lecanicillium attenuatum against cotton aphid. J Asia Pac Entomol 11:1–4
Kim JJ, Roberts DW (2012) The relationship between conidial dose, moulting and insect developmental stage on the susceptibility of cotton aphid, Aphis gossypii, to conidia of Lecanicillium attenuatum, an entomopathogenic fungus. Biocontrol Sci Technol 22:319–331
Leite LG, Alves SB, Batista Filho A, Roberts DW (2005) Simple, inexpensive media for mass production of three entomophthoralean fungi. Mycol Res 109:326–334
Liu Y-Q, Zhang F-C, Liu S-S (2003) Effect of moulting in Myzus persicae on the virulence of the entomopathogenic fungus Beauveria bassiana. Acta Entomol Sinica 46:441–446
Lopez DC, Zhu-Salzman K, Ek-Ramos MJ, Sword GA (2014) The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 9(8):e103891
López-Sandin I, Gutiérrez-Soto G, Gutiérrez-Díez A, Medina-Herrera N, Gutiérrez-Castorena E, Galicia-Juárez M, Zavala-García F (2021) Biomass and sugar production dynamics in sweet sorghum variety Roger. Chil J Agr Res 81(1):92–101. https://doi.org/10.4067/S0718-58392021000100092
Loureiro EDS, Moino Jr A (2006) Patogenicidade de fungos hifomicetos aos pulgões Aphis gossypii Glover e Myzus persicae (Sulzer)(Hemiptera: Aphididae). Neotrop Entomol 35:660–665
Mantzoukas S, Lagogiannis I (2019) Endophytic Colonization of Pepper (Capsicum annuum) controls Aphids (Myzus persicae Sulzer). SI: Endophytic Entomopathogenic Fungi: New approach for controlling serious pests. Appl Sci 9(11):2239. https://doi.org/10.3390/app9112239
Mantzoukas S, Eliopoulos P (2020) Endophytic entomopathogenic fungi: a valuable biological control tool against plant pests. Applied Science, SI: Endophytic Entomopathogenic Fungi: New approach for controlling serious pests. Appl Sci 10(1):370. https://doi.org/10.3390/app10010360
Mesquita A, Lacey LA, Mercadier G, LeClant F (1996) Entomopathogenic activity of a whitefly derived isolate of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against the Russian wheat aphid, Diuraphis noxia (Homoptera: Aphididae) with the description of an effective bioassay method. Eur J Entomol 93:69–75
Milner RJ (1997) Prospects for biopesticides for aphid control. Entomophaga 42:227–239
Poveda J (2021) Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol Control 159:104634. https://doi.org/10.1016/j.biocontrol.2021.104634
Ramegowda GK, Vidya M, Patil RK, Puttannavar MS, Lingappa S (2007) Laboratory and field evaluation of some entomopathogenic fungi against sugarcane woolly aphid, Ceratovacuna lanigera Zehntner (Homoptera: Aphididae). J Biol Control 21:173–176
SENASICA., https://www.gob.mx/cms/uploads/attachment/file/523736/Pulg_n_amarillo_del_sorgo_ENG.pdf
Shahid AA, Rao AQ, Bakhsh A, Husnain T (2012) Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch Biol Sci 64:21–42. doi:https://doi.org/10.2298/ABS1201021S
Shan LT, Feng MG (2010) Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera:Aphididae). Pest Manag Sci 66:669–675
Shaw KE, Davidson G, Clark SJ, Ball BV, Pell JK, Chandler D, Sunderland KD (2002) Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol Control 24:266–276. doi:https://doi.org/10.1016/S1049-9644(02)00029-4
Sierotzki H, Wullschleger J, Gisi U (2000) Point mutation in cytochrome b gene conferring resistance to strobilurin fungicides in Erysiphe graminis f. Sp. Tritici field isolates. Pestic Biochem Physiol 68:107–112. doi:https://doi.org/10.1006/pest.2000.2506
Tesfaye D, Seyoum E (2010) Studies on the pathogenicity of native entomopathogenic fungal isolates on the cotton/melon aphid, Aphis gossypii (Homoptera: Aphididae) Glover under different temperature regimes. Afr Entomol 18:302–312
Vandenberg J (1996) Standardized bioassay and screening of Beauveria bassiana and Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against Russian wheat aphid (Homoptera: Aphididae). J Econ Entomol 89:1418–1423
Vu VH, Hong SI, Kim K (2007) Selection of entomopathogenic fungi for aphid control. J Biosci Bioeng 104:498–505
Wakil W, Brust GE, Perring TD (eds) 2017. Sustainable management of arthropod pests of tomato.Academic Press
Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ (2003) Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci 59:156–165
Zaki FN (1998) Efficiency of the entomopathogenic fungus, Beauveria bassiana (Bals), against Aphis crassivora Koch and Bemesia tabaci. Gennandius J Appl Entomol 122:397–399
Acknowledgements
The authors thank Ana Patricia Barajas Morin, Denisse Abril Aguilar Morales, Liliana Elizabeth Gonzalez Lopez, Edith Alejandra Cordova Rojo, Christian Aimee Gonzalez Luna, Jesus Oziel Zuñiga Sanchez, Adan Alejandro Galindo Campos, Eduardo Daniel Hernandez Ruiz and Javier Sanchez for their valuable support during the sorghum field trial in Mexico.
Funding
This study was supported by grants: PRODEP- UANL-PTC-849 and PAICYT- CT242-15 to MJER. We thank SNI-CONACYT support 16614 to PTG and 54340 to MJER.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study´s conception and design. Material preparation, data collection and analysis were performed by MJER, SM and IL. The first draft of the manuscript was written by SM and MJER and all authors commented on previous versions of the manuscript and improved them until reaching the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
No conflict of interest or competing interests have been declared by all authors.
Ethical approval
was waived by the local Ethics Committee of the Autonomous University of Nuevo Leon and the University of Ioannina, in view of the nature of the study in the field on natural aphids’ populations, whereas all the procedures being performed were part of the routine care followed in the rearing and bioassays using the aphids’ laboratory colonies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mantzoukas, S., Tamez-Guerra, P., Zavala-Garcia, F. et al. Entomopathogenic fungi tested in planta on pepper and in field on sorghum, to control commercially important species of aphids. World J Microbiol Biotechnol 38, 84 (2022). https://doi.org/10.1007/s11274-022-03268-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03268-7