Abstract
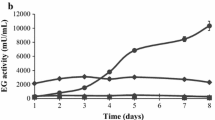
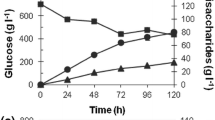
BglG, a Stachybotrys microspora β-glucosidase produced in the presence of glucose and cellobiose, was used individually as sole carbon source. The time course synthesis of BglG showed two aspects: (1) an exponential curve, observed in glucose Mandels medium, and (2) a cloche curve, observed in cellobiose containing cultures. A decrease was observed in bglG production at the 6th, 8th and 10th days during mycelium growth in cellobiose Mandels medium, which allowed for the assumption that the anabolism of a bglG inhibitor factor was produced with cellobiose but not with glucose. Cellobiose dehydrogenases (CDH) activity was, on another hand detected in cellobiose grown cultures but not in glucose containing ones. The aliquots, withdrawn at the time course of bglG production in the presence of cellobiose, gave rise to an inhibitory effect on bglG activity. This result was obtained with and without the heat treatment (5 min at 100°C) of the aliquots, which supported the non-proteinaceous nature of the inhibitor factor. Furthermore, sugar chromatographic analyses revealed the appearance of a secondary metabolite in the cellobiose Mandels medium and indicated that the factor behind the bglG activity cloche curve was a δ-gluconolactone. Seeing that the latter follows a strong inhibitory effect on bglG activity, it is speculated that the decrease in bglG activity during the time course of bglG synthesis in cellobiose Mandels medium is assigned to the release of δ-gluconolactone. This paper presents and validates an explanatory model for this hypothesis.








Similar content being viewed by others
References
Amouri B, Gargouri A (2006) Characterization of a novel [beta]-glucosidase from a Stachybotrys strain. Biochem Eng J 32:191–197
Baldrian P, Valaskova V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32:501–521
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities utilizing the principal of protein dye binding. Anal Biochem 72:248–254
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301–308
Brini F, Saibi W, Amara I, Gargouri A, Masmoudi K, Hanin M (2010) Wheat dehydrin DHN-5 exerts a heat-protective effect on beta-glucosidase and glucose oxidase activities. Biosci Biotechnol Biochem 74:1050–1054
Buaban B, Inoue H, Yano S, Tanapongpipat S, Ruanglek V, Champreda V, Pichyangkura R, Rengpipat S, Eurwilaichitr L (2010) Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J Biosci Bioeng 110:18–25
Davies T (2010) Glycosidase inhibition: assessing mimicry of the transition state. Org Biomol Chem 8:305–320
Hardiman E, Gibbs M, Reeves R, Bergquist P (2010) Directed evolution of a thermophilic β-glucosidase for cellulosic bioethanol production. Appl Biochem Biotechnol 161:301–312
Harreither W, Sygmund C, Augustin M, Narciso M, Rabinovich ML, Gorton L, Haltrich D, Ludwig R (2011) Cellobiose dehydrogenases from ascomycetes: catalytic properties and classification AEM. 02052-02010
Henriksson G, Polk V, Eriksson KEL (1997) Assay for cellobiose dehydrogenase in the presence of laccase. Biotech Tech 11:743–745
Ishida T, Fushinobu S, Kawai R, Kitaoka M, Igarashi K, Samejima MF (2009) Crystal structure of glycoside hydrolase family 55 {beta}-1, 3-glucanase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem 284:10100–10109
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Lever M, Ho G, Cord-Ruwisch R (2010) Ethanol from lignocellulose using crude unprocessed cellulase from solid-state fermentation. Bioresour Technol 101:7083–7087
Li B, Nagalla SR, Renganathan V (1997) Cellobiose dehydrogenase from Phanerochaete chrysosporium is encoded by two allelic variants. Appl Environ Microbiol 63:796–799
Lin J, Ndlovu LM, Singh Sand Pillay B (1999) Purification and biochemical characteristics of beta-d-xylanase from a thermophilic fungus, Thermomyces lanuginosus-SSBP. Biotechnol Appl Biochem 30(Pt 1):73–79
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol 73:269–278
Pozzo T, Pasten JL, Karlsson EN, Logan DT (2010) Structural and functional analyses of beta-glucosidase 3B from Thermotoga neapolitana: a thermostable three-domain representative of glycoside hydrolase 3. J Mol Biol 397:724–739
Saibi W, Amouri B, Gargouri A (2007) Purification and biochemical characterization of a transglucosilating beta-glucosidase of Stachybotrys strain. Appl Microbiol Biotechnol 77:293–300
Saibi W, Abdeljalil S, Gargouri A (2010) Carbon source directs the differential expression of β-glucosidases in Stachybotrys microspora. World J Microbiol Biotechnol. doi:10.1007/s11274-010-0634-x
Smaali MI, Michaud N, Marzouki N, Legoy MD, Maugard T (2004) Comparison of two beta-glucosidases for the enzymatic synthesis of beta-(1–6)-beta-(1–3)-gluco-oligosaccharides. Biotechnol Lett 26:675–679
Acknowledgments
This work was supported by grants from the Ministry of Higher Education, Scientific Research and Technology, Tunisia. The authors wish to express their gratitude to Pr. ANOUAR Smaoui from the Sfax Faculty of Science for his constructive proofreading and language polishing services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saibi, W., Gargouri, A. Cellobiose dehydrogenase influences the production of S. microspora β-glucosidase. World J Microbiol Biotechnol 28, 23–29 (2012). https://doi.org/10.1007/s11274-011-0787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0787-2