Abstract
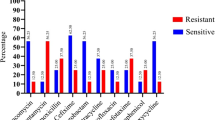
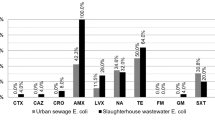
We investigated for the first time the occurrence, stability and antibiotic resistance of 593 enterococci in six samples collected from three urban sewage treatment plants (STPs) located in the north, south and west part of Tehran, Iran between October 2004 and September of 2005. Isolates were typed with a biochemical fingerprinting method (the PhPlate system) and tested for their resistance to six antibiotics. The most prevalent species in all three STPs were E. faecium followed by E. hirae and E. faecalis accounting for 93% of the total isolates examined. In all, 317 (55%) isolates were susceptible to all six antibiotics tested and the remaining isolates were resistant to between 1 and 6 antibiotics. Biochemical fingerprinting with PhPlate system showed a high diversity for E. faecalis (D i = 0.95), E. hirae (D i = 0.93) and E. faecium (D i = 0.95) populations with an overall diversity of D i = 0.97 for the whole enterococcal populations found in all three STPs. Our data indicate a high degree of polyclonality among the enterococci populations of human origin. This study suggest that the municipal wastewaters might be an important source of dissemination of antibiotic-resistant enterococci in Iran.



Similar content being viewed by others
References
Aarestrup, F. M., Agerso, Y., Gerner-Smidt, P., Madsen, M., & Jensen, L. B. (2000). Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagnostic Microbiology and Infectious Disease, 37, 127–137.
Anonymous. (1998). Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association, American Works Association, Water Environment Federation.
Blanch, A. R., Caplin, J. L., Iversen, A., Kühn, I., Manero, A., Taylor, H. D., et al. (2003). Comparison of enterococcal populations related to urban and hospital wastewater in various climatic and geographic European regions. Journal of Applied Microbiology, 94, 994–1002.
Bonten, M. J., Hayden, M. K., Nathan, C., Rice, T. W., & Weinstein, R. A. (1998). Stability of vancomycin-resistant enterococcal genotypes isolated from long-term colonized patients. Journal of Infectious Diseases, 177, 378–382.
Borgen, K., Sorum, M., Wasteson, Y., Kruse, H., & Oppegaard, H. (2002). Genetic linkage between erm(B) and vanA in Enterococcus hirae of poultry origin. Microbial Drug Resistance, 8, 363–368.
Butaye, P., Devriese, L. A., & Haesebrouck, F. (2001). Differences in antibiotic resistance patterns of Enterococcus faecalis and Enterococcus faecium strains isolated from farm and pet animals. Antimicrobial Agents and Chemotherapy, 45, 1374–1378.
Costa, P. M., Vaz-Pires, P., & Bernardo, F. (2006). Antimicrobial resistance in Enterococcus spp. Isolated in inflow, effluent and sludge from municipal sewage water treatment plants. Water Research, 40, 1735–1740.
Facklam, R. R., & Collins, M. D. (1998). Identification of Enterococcus species isolated from human infections by a conventional test scheme. Journal of Clinical Microbiology, 27, 731–734.
Gambarotto, K., Ploy, M. C., Turlaure, P., Grelaud, C., Martin, C., Bordessoule, D., et al. (2000). Prevalence of vancomycin-resistant Enterococci in fecal samples from hospitalized patients and nonhospitalized controls in a cattle-rearing area of France. Journal of Clinical Microbiology, 38, 620–624.
Harwood, J. V., Brownell, M., Perusek, W., & Whitlock, E. J. (2001). Vancomycin-resistant Enterococcus spp. isolated from wastewater and chicken feces in the United States. Applied and Environmental Microbiology, 67, 4930–4933.
Iversen, A., Kühn, I., Franklin, A., & Möllby, R. (2002). High prevalence of vancomycin-resistant Enterococci in Swedish sewage. Applied and Environmental Microbiology, 68, 2838–2842.
Jackson, C. R., Fedorka-Cray, P. J., & Barrett, J. B. (2004). Use of genus- and species-specific Multiplex PCR for identification of enterococci. Journal of Clinical Microbiology, 42, 3558–3565.
Juan, S. A., Loyola, P., Galleguillos, J., Rodriguez, Y., Coque-Navarro, P., Möllby, R., et al. (2005). Prevalence of antibiotic resistant Enterococcus spp in waste waters in the North of Chile. Revista Médica de Chile, 133, 1201–1210.
Kühn, I., Iversen, A., Burman, L. G., Olsson-Liljequist, B., Franklin, A., Finn, M., et al. (2000). Epidemiology and ecology of enterococci, with special reference to antibiotic resistant strains, in animals, humans and the environment. Example of an ongoing project within the European research programme. International Journal of Antimicrobial Agents, 14, 337–342.
Kühn, I., Iversen, I., Finn, M., Greko, C., Burman, L. G., Blanch, A. R., et al. (2005). Occurrence and relatedness of vancomycin-resistant Enterococci in animals, humans, and the environment in different European regions. Applied and Environmental Microbiology, 71, 5383–5390.
Kühn, I., Iversen, A., & Möllby, R. (2003). The PhenePlate system for studies of the diversity of enterococcal populations from the food chain and the environment. International Journal of Food Microbiology, 88, 189–196.
Laukova, A., & Juris, P. (1997). Distribution and characterization of Enterococcus species in municipal sewages. Microbios, 89, 73–80.
Liassine, N., Frei, R., Jan, I., & Auckenthaler, R. (1998). Characterization of glycopeptide-resistant enterococci from Swiss hospital. Journal of Clinical Microbiology, 36, 1853–1858.
Manson, J. M, Keis, S., Smith, J. M., & Cook, G. M. (2003). A clonal lineage of vanA-type Enterococcus faecalis predominates in vancomycin-resistant Enterococci isolated in New Zealand. Antimicrobial Agents and Chemotherapy, 47, 204–210.
Manson, J. M., Smith, J. M. B., & Cook, G. M. (2004). Persistence of vancomycin-resistant Enterococci in New Zealand broilers after discontinuation of avoparcin use. Applied and Environmental Microbiology, 70, 5764–5768.
Murray, B. E. (1998). Diversity among multidrug-resistant enterococci. Emerging Infectious Diseases, 4, 37–47.
Nallapareddy, S. R., Wenxiang, H., Weinstock, G. M., & Murray, E. (2005). Molecular characterization of a widespread, pathogenic, and antibiotic resistance receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. Journal of Bacteriology, 187, 5709–5718.
National Committee for Clinical Laboratory Standards. (2001). Performance standards for antimicrobial susceptibility testing, 11th informational supplement, vol. 21. Wayne, Pa. USA: National Committee for Clinical Laboratory Standards.
Novais, C., Couque, T. M., Ferreira, H., Sousa, J. C., & Peixe, L. (2005). Environmental contamination with vancomycin-resistant enterococci from hospital sewage in Portugal. Applied and Environmental Microbiology, 71, 3364–3368.
Robredo, B., Singh, K., Baquero, F., Murray, B. E., & Torres, C. (2000). Vancomycin-resistant enterococci isolated from animals and food. International Journal of Food Microbiology, 54, 197–204.
Simjee, S., White, D. G., McDermott, P. F., Wagner, D. D., Zervos, M. J., Donabedian, S. M., et al. (2002). Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections. evidence of gene exchange between human and animal enterococci. Journal of Clinical Microbiology, 40, 4659–4665.
Sneath, P. H. A., & Sokal, R. R. (1973). Numerical taxonomy. San Fransisco: The W. H. Freeman Co.
Svec, P., & Sedlacek, I. (1999). Occurrence of Enterococcus spp. in waters. Folia Microbiologica, 44, 3–10.
Yost, C. K., & Nattress, F. M. (2000). The use of multiplex PCR reactions to characterize populations of lactic bacteria associated with meat spoilage. Letters in Applied Microbiology, 31, 129–133.
Acknowledgments
This work was supported in part by World Health Organization, Eastern Mediterranean Regional Office grant no: R6/18/3, Swedish international development cooperation agency (Sida) grant no: 6342 and Ministry of Health of Iran, under-secretariat of research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talebi, M., Rahimi, F., Katouli, M. et al. Prevalence and Antimicrobial Resistance of Enterococcal Species in Sewage Treatment Plants in Iran. Water Air Soil Pollut 185, 111–119 (2007). https://doi.org/10.1007/s11270-007-9435-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9435-8