Abstract
The zoonotic bacterium Coxiella (C.) burnetii can be excreted by infected goats through birth products and milk. The detection of C. burnetii DNA in the mammary gland tissue of infected dairy goats and intermittent milk shedders has been reported, but confirmation of C. burnetii bacteria in the udder remained pending. The pathogen caused abortions in a 152-head dairy goat herd, resulting in the vaccination against C. burnetii of the entire herd with annual boosters. To monitor the C. burnetii shedding at herd level, monthly bulk tank milk (BTM) samples were analyzed using PCR (IS1111). Despite vaccination, C. burnetii DNA was detected in BTM samples within the first 16 months of the study. Therefore, individual milk samples were tested on four different occasions several months apart to identify potential intermittent milk shedders. Only one goat (#67455) tested positive three times. This goat was necropsied to investigate the presence of C. burnetii in the udder and other organs. PCR detected C. burnetii DNA solely in both mammary glands and the left teat cistern. Immunohistological examination identified C. burnetii antigen in mammary gland tissue, confirmed by the detection of C. burnetii bacteria in the mammary epithelial cells using fluorescence in situ hybridization. The removal of goat #67455 led to negative BTM samples until the end of the study. The findings demonstrate the occurrence of C. burnetii in the mammary gland of a naturally infected and vaccinated goat. The presence possibly contributed to intermittent milk shedding of goat #67455, and the mammary gland tissue may serve as a replicative niche for C. burnetii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Q fever is a worldwide zoonosis caused by the obligate intracellular bacterium Coxiella (C.) burnetii. Ruminants are considered the primary reservoir and the pathogen replicates particularly in the trophoblasts of the placenta, with the rate of replication increasing toward parturition (Celina and Cerný 2022; Roest et al. 2012). Up to 109 organisms per gram may be present in one gram of placental tissue (Arricau-Bouvery et al. 2003). Infected animals may suffer from reproductive disorders and excrete the bacteria during abortion or parturition through birth products (placenta, amniotic fluid), but also through milk and feces (Bauer et al. 2020; Ullah et al. 2022). In experimentally infected goats, C. burnetii DNA was detected in milk between 38 and 52 days after parturition (Roest et al. 2012). In contrast, bulk tank milk (BTM) from naturally infected dairy goat herds remained C. burnetii positive for several months (Álvarez-Alonso et al. 2018; Bauer et al. 2022). Therefore, monitoring dairy goat herds with BTM samples analyzed by ELISA or PCR has been proven to be effective to identify C. burnetii-positive herds (Jansen et al. 2021; van den Brom et al. 2012; Vellema et al. 2021). Both methods can detect a within-herd prevalence of at least 15% (van den Brom et al. 2012). Given that these methods operate on distinct principles, the concordance between the two assays is satisfactory, despite the ELISA showing reduced sensitivity and specificity compared to the PCR (van den Brom et al. 2012). Intermittently C. burnetii shedding goats resulted in discontinuously positive BTM samples, even though the animals were vaccinated (Boarbi et al. 2014; van den Brom et al. 2013). Therefore, it is important to identify intermittent shedders to reduce the risk of transmission. Although, the route of transmission through milk is still not fully understood but experimental studies in the past have shown successful C. burnetii infection through the teat canal (Williams 1991). In recent years the pathogen was detected, using PCR methods, in the mammary gland and mammary lymph nodes in goats (Roest et al. 2012; Sánchez et al. 2006; van den Brom et al. 2013). However, the conclusive evidence of the occurrence of C. burnetii in the caprine mammary gland tissue through histopathological techniques such as immunohistochemistry and fluorescence in situ hybridization is still pending. The detection of C. burnetii DNA in milk and udder tissue from naturally and experimentally infected goats, along with the propagation of C. burnetii isolates from goats in bovine mammary gland epithelial cells in vitro, raises the question whether the udder acts as replicative niche for C. burnetii (Roest et al. 2012; Sánchez et al. 2006; Sobotta et al. 2022; van den Brom et al. 2013).
The world’s largest human Q fever outbreak was recorded in The Netherlands and was associated with large dairy goat farms in the south of the country. From 2007 to 2011, more than 4,000 people contracted Q fever and the number of infected people is estimated to be about 40,000 (Reedijk et al. 2013; van Roeden et al. 2018). The main route of infection for humans and animals is inhalation of contaminated aerosols. In humans, approximately 40% of infected individuals show flu-like symptoms such as fever, headache, myalgia and pneumonia (Eldin et al. 2017). In the long term, up to 20% of patients with acute Q fever develop chronic fatigue syndrome (Morroy et al. 2016), and affected patients with cardiovascular lesions suffer from endocarditis and vascular disease (Eldin et al. 2017). The clinical impact of consuming raw milk and raw milk products contaminated with C. burnetii is still under debate (Barandika et al. 2019; Pexara et al. 2018). A study of prison inmates demonstrated that consumption of raw milk contaminated with C. burnetii resulted in seroconversion but not the development of clinical symptoms (Benson et al. 1963). Macrophages are the primary target cells of C. burnetii, and it is assumed that the higher numbers in the lungs compared to the gastrointestinal tract makes infection with C. burnetii by inhalation more effective than oral ingestion of the pathogen (Gale et al. 2015). Nevertheless, regular consumption of raw milk containing C. burnetii has resulted in Q fever symptoms in a few cases (Fishbein and Raoult 1992; Signs et al. 2012). Dupont and colleagues (1992) even suggest a link between raw milk consumption and C. burnetii-associated hepatitis. Currently, the risk of contracting Q fever from consuming raw milk and raw milk products is considered to be low but not negligible (Gale et al. 2015; Pexara et al. 2018). Due to the increasing popularity of raw milk consumption and new ways of selling raw milk through vending machines and internet sales (EFSA Panel on Biological Hazards 2015), there is a need to raise the awareness of food-borne pathogens in these products. The process of high-temperature short-time pasteurization inactivates C. burnetii in milk (Wittwer et al. 2022).
For the prevention and control of Q fever in goat herds, an inactivated C. burnetii Phase I vaccine (Coxevac®, Ceva Santé Animale, Libourne, France) is licensed in several European countries for cattle, goats, and recently for sheep. C. burnetii shedding is greatly reduced, but not completely prevented, when goats are vaccinated before infection occurs (Arricau-Bouvery et al. 2005). The vaccine is also unable to prevent excretion both during a Q fever outbreak and in the subsequent kidding season (Bauer et al. 2020, 2022; De Cremoux et al. 2012).
The primary objective of the study was to monitor the shedding of C. burnetii at the herd level using BTM samples from a highly infected dairy goat herd that was subsequently vaccinated. Despite applying a strict vaccination protocol, the BTM samples continued to test positive for C. burnetii DNA using PCR. Therefore, the second study objective was to identify potential intermittent milk shedders by analyzing individual milk samples and to detect the presence of C. burnetii bacteria in the mammary gland tissue.
Material & methods
Herd history
A dairy goat herd with 152 goats in the German federal state of North Rhine-Westphalia reported abortions, stillbirths and weak kids at the end of the kidding season in April 2018. Four aborted fetuses with placentas from two goats were examined by the North Rhine-Westphalia state laboratory to determine the cause of abortion. The samples were tested for the presence of Brucella spp, Campylobacter spp., Chlamydia spp., C. burnetii, bluetongue virus, pestivirus and Schmallenberg virus. The only abortifacient pathogen detected was C. burnetii (Cq 11–22, VetMAX™ C. burnetii Absolute Quant Kit, Thermo Fisher Scientific GmbH, Dreieich, Germany). The C. burnetii-DNA obtained from the placenta was further analyzed with MLVA/VNTR genotyping as previously described (Frangoulidis et al. 2014), and genotype associated with the German sheep population, A3, was identified. The farmer asked the Clinic for Swine and Small Ruminants at the University of Veterinary Medicine Hannover, Foundation, Hannover, Germany, for help in controlling the Q fever outbreak in her dairy goat herd. More details about the Q fever outbreak have been published elsewhere (Bauer et al. 2022).
Vaccination
Shortly after the detection of C. burnetii in April 2018, all adult goats were vaccinated twice at three-week intervals with an inactivated C. burnetii Phase I vaccine (Coxevac®, Ceva, Libourne, France) according to the manufacturer’s instructions. A booster vaccination was given to all adult goats in July 2019 and July 2020, and the female progeny received their primary vaccination (as described above) four weeks before the start of the breeding season.
Monitoring
To monitor C. burnetii excretion in milk at herd level, monthly BTM samples were collected from April 2018 until December 2021, with one sample missing in September 2020. Due to the irregular detection of C. burnetii DNA in the BTM, individual milk samples were taken from the continuously milked goats (n = 96) in January 2020 and colostrum samples (< 24 h post-partum) were collected from freshly kidded goats (n = 69) during kidding season 2020 (March-April 2020). This sampling period is defined as “Spring Sampling 2020”. Individual milk samples were taken again from all lactating goats in July 2020 (n = 162) and November 2020 (n = 150). Pregnant goats were dried off six weeks before the start of kidding in 2021. Again, all continuously milked goats were sampled by individual milk samples in March 2021 (n = 93) and colostrum was taken from freshly kidded goats (n = 49) during kidding season 2021 (March-April 2021). The sample collection in March 2021 and during kidding season 2021 represent the “Spring Sampling 2021”. The number of goats sampled varied on each sampling date due to animal losses or sales. The colostrum and milk samples were collected from both mammary glands from the same goat, under aseptic conditions, in one milk tube and stored at -20 °C until further analysis.
Dairy goat #67455
C. burnetii DNA was detected in individual milk samples from dairy goat #67455(born January 2013) on three of the four sampling dates. This goat gave birth to healthy triplets on 26th February 2018 and was milked continuously until removed from the herd. The goat received its primary vaccination in 2018 and was boostered twice in July 2019 and 2020. Only this goat was included in further examinations. From 20th May to 16th June 2021 (28-day period), individual milk samples from goat #67455 were collected separately from both mammary glands before the start of morning milking and under aseptic conditions. The specimens were stored at -20 °C until further PCR analysis. Moreover, sterile milk samples (sterile plastic tubes with boric acid, KABE-Labortechnik GmbH, Nümbrecht, Germany) were taken from each mammary gland to determine somatic cell counts (SCC) and microbiological composition before the goat was euthanized. In addition, a serum sample was tested for antibodies against caprine arthritis encephalitis virus (CAEV) using a commercial ELISA according to the manufacturer’s recommendations (IDVet, Grabels, France) and gave a negative result. The goat was euthanized with an intravenous injection of sodium pentobarbital (Euthadorm®, CP-Pharma GmbH, Burgdorf, Germany) and the necropsy was performed on the same day at the North Rhine-Westphalia state laboratory. A complete necropsy was performed, strictly avoiding contamination with milk. The udder was cut open last, taking strict care not to mix the milk from both halves of the udder. Tissue samples were taken from both lactiferous glands. One specimen was collected bilaterally from each udder, including lymph nodes, mammary gland tissue, lactiferous ducts, gland cistern, teat cistern, and teat canal. Additionally, tissue samples were taken from the reproductive tract (ovaries, oviducts, uterine horns, uterus, and lymph nodes). A new sterile biopsy punch (Ø 8 mm, Kruuse, Langeskov, Denmark) was used for each tissue sample from both lactiferous glands and the reproductive tract. The biopsy punches were transferred to plastic tubes with screw caps before molecular analysis to prevent cross-contamination with C. burnetii. Swab samples from the uterus and vagina were collected. Specimens from the hematopoietic system (spleen, thymus), liver, urinary tract (renal pelvis, urine), respiratory tract (lymph nodes, lungs), cerebrospinal fluid and feces were also included in the examinations (Table 1). The non-tissue samples and one sample of each tissue specimen were stored at -20 °C for PCR analysis, and a second tissue sample was fixed in 10% phosphate buffered formalin for histopathology.
Laboratory examination
Molecular analysis of milk and tissue samples
The BTM samples were prepared for PCR analysis as follows:
2 mL of the BTM was centrifuged for 5 min at 2655 g. Subsequently, the fat was removed from the tube with a sterile swab. After another centrifugation step for 10 min at 20,817 g, the supernatant was disposed of. Bacterial DNA was prepared from the remaining pellet using the InviMag® Universal Kit/ KF96 (STRATEC Molecular GmbH, Berlin, Germany) according to the manufacturer’s instructions.
In the case of tissue samples, 20 mg of each sample was mixed with 400 µL molecular biology grade water and then crushed with a steel bullet using the TissueLyser® (QIAGEN, Venlo, Netherlands) for 2 min at 15 Hz. Afterwards 200 µL were put in the InviMag® Universal Kit/ KF96 (STRATEC Molecular GmbH, Berlin, Germany) and the bacterial DNA was prepared according to the manufacturer’s instructions.
The extracted DNA from the BTM samples were analyzed with a commercially available real-time PCR (LSI VetMAX™ C. burnetii Absolute Quant Kit, Thermo Fisher Scientific GmbH, Dreieich, Germany), which targets IS1111. The manufacturer indicates Cq values ≤ 45 as positive. C. burnetii-specific DNA fragments in the individual milk, colostrum and organ samples were detected by amplification of the IS1111 elements with an in-house real-time PCR according to Frangoulidis and colleagues (2012) due to limited resources. Cycle Quantification (Cq) values ≤ 45 were indicated as positive and Cq values > 45 as negative values. The detection limit for both real-time PCRs is 1 genome equivalent (GE) per PCR evaluated with the Nine Mile genome containing 20 copies of IS1111 per GE.
Cytological and microbiological analysis of the milk samples
Both milk samples collected shortly before euthanasia of goat #67455 were sent for routine cytological and bacteriological analysis at a specialized laboratory (MBFG, Wunstorf, Germany), to identify any bacteria which may cause mastitis, which could interfere with the histopathological findings from the udder tissue. In brief, the somatic cell count (SCC) of the milk was determined using a fluorescence-based optical method (Fossomatic™ 360, Type 15,700, Foss, Hilleroed, Denmark), and microbiological analysis was performed by incubating a milk smear on Aesculin Blood Agar (Oxoid Deutschland GmbH, Wesel, Germany) for 48 h at 37 °C under aerobic conditions.
Histopathology and immunohistochemistry
For histology, several tissue samples from both mammary glands were examined. Immunohistochemistry (IHC) was performed on both mammary gland tissues and the left teat cistern due to positive PCR results (Table 1). The tissues were fixed, dehydrated, cleared, and processed into paraffin wax blocks. Sections of 3 μm were cut and routinely stained with hematoxylin/eosin and immunostained for the presence of C. burnetii antigen. Immunohistochemical staining of C. burnetii antigen was performed as described previously (Baumgärtner et al. 1988). Briefly, dewaxed and rehydrated tissue sections were incubated with a polyclonal antibody rabbit antibody specific for C. burnetii, followed by a biotinylated goat-anti-rabbit antibody and the avidin biotin peroxidase complex (ABC method Vectastain®, Vector Laboratories, Burlingame, CA, USA) according to the instructions of the manufacturer. The immunohistological reaction was visualized using 3,3´-diaminobenzidine-tetrahydrochloride (DAB) as chromogen. Tissues from mice experimentally infected with C. burnetii were used as a positive control (Baumgärtner et al. 1988).
Fluorescent in situ hybridization (FISH)
FISH was performed on 3 µm thick tissue sections from the left and right mammary gland tissue according to Buijs et al. (2022). Briefly, four oligonucleotide RNA-probes (S-S-C.burnetii-188, S-S-C.burnetii-631, S-S-C.burnetii-826, and S-S-C.burnetii-1462) targeting different locations of the 16S ribosomal RNA of C. burnetii, were used in a mixture: The oligonucleotide probes were labeled at the 5’ and 3’ end with fluorescein isothiocyanate (FITC) (green) (Eurofins MWG Operon, Ebersberg, Germany). Hybridization was performed at 45 °C with 40 µL of hybridization buffer (100 mM Tris [pH 7.2], 0.9 M NaCl, 0.1% sodium dodecyl sulphate) and 200 ng of each probe for at least 16 h in a Sequenza slide rack (ShandonTM, Thermo Fisher Scientific, Rosklide, Denmark). After hybridization, sections were washed three times with hybridization buffer at 45 °C for 15 min and subsequently three times with washing buffer (100 mM Tris [pH 7.2], 0.9 M NaCl). Sections were rinsed in water, air dried, and mounted in Vectashield (Vector Laboratories, Newark, CA, United States) for fluorescence microscopy. An Axioplan2 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany) equipped with a 100-W HBO lamp and filter sets 24, 38 and 43 was used to examine the hybridized specimens. Positive identification of C. burnetii was based on a specific hybridization signal from coccoid intracytoplasmic organisms. Images were obtained using an AxioCam MRm version 3 FireWire monochrome camera and the AxioVision software, version 4.5 (Carl Zeiss, Oberkochen, Germany). Tissue sections from a case of spontaneous bovine placenta infection with C. burnetii were used as a positive control (Wolf-Jäckel et al. 2021).
Statistical analysis
The PCR results of the daily collected milk samples (20th May to 16th June 2021) from goat #67455 were analyzed using a Mann-Whitney U test (GraphPad Prism 9, Cypress, CA, USA). A result of p < 0.05 was considered significant.
Results
Bulk tank milk samples
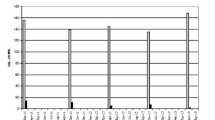
After C. burnetii was diagnosed in the dairy goat herd, BTM samples remained positive for 16 months. Thereafter, the results showed an undulating trend with negative outcomes from October 2019 until February 2020. In June 2021, the intermittent shedder (goat #67455) was removed from the herd and subsequent monthly BTM specimens tested negative until the end of the study. The results are shown in detail in Fig. 1.
Monthly detection of C. burnetii DNA (Cq ≤ 45) in bulk tank milk (BTM) determined by real-time PCR (IS1111) from a naturally infected dairy goat herd (April 2018 until December 2021). The syringes symbolize the dates of vaccination against C. burnetii. Tubes indicate the sampling of individual milk specimens from goats. In June 2021, goat #67455, which shed C. burnetii intermittently, was removed from the herd and was necropsied. BTM in September 2020 is missing
Individual milk samples
Eleven goats tested positive for C. burnetii in individual milk samples during the four sampling periods/dates. The eleven goats had already been involved in the C. burnetii infection in 2018. Of these, ten goats contained C. burnetii DNA in their milk only once. Goat #67455 was the only animal that tested positive for C. burnetii on three of the four sampling dates. This goat was continuously milked and no colostrum specimens were available in the 2020 and 2021 kidding seasons. Details of the detection of C. burnetii DNA in individual milk samples are illustrated in Fig. 2.
C. burnetii DNA in individual milk samples from different sampling periods/dates. Figures in parentheses represent numbers of sampled animals. Only goat #67455 tested positive several times (triangle). All other positive tested goats shed C. burnetii once. The dashed line indicates the cut-off value of the PCR assay (Cq ≤ 45)
Individual milk samples goat #67455
During the 28-day study period, all individual milk samples from the left mammary gland of goat #67455 tested positive for C. burnetii DNA. Twenty-five samples from the right mammary gland contained C. burnetii DNA (Fig. 3). There was no significant difference in the obtained Cq values between the two mammary glands.
Cytological and microbiological results
The SCC of the left and right udder half from goat #67455 were 299 × 103 cells/ml and 270 × 103 cells/ml, respectively, on the day of euthanasia (22nd June 2021). No pathogens were detected by the cultural bacteriological examination.
Molecular analysis of tissue samples
DNA of C. burnetii was detected in both samples from both mammary tissues and in the left teat cistern. All other tissue samples tested negative by real-time PCR (Table 1).
Histopathology and immunohistochemistry
Histological examination of the mammary gland tissue of both udder halves revealed multifocal mild, predominantly lymphoplasmacytic interstitial inter-, intralobular and periductual infiltrations (Fig. 4). In alveoli and ducts of the gland, there were several corpora amylacea focally surrounded by multinuclear macrophages.
A. Lactating mammary gland with interstitial lymphocytic, focally granulomatous inflammation surrounding a corpus amylaceum (asterisks) characterized by macrophages and single multinucleated giant cell (arrow). B. Lymphoplasmacytic infiltrates (arrowheads) in the interalveolar interstitium, HE-Staining
In both mammary gland tissues, single epithelial cells revealed cytoplasmic granular labeling (Fig. 5). The left teat cistern showed no immunolabeling.
Fluorescent in situ hybridization (FISH)
C. burnetii bacteria were identified in the mammary gland tissue by FISH (Fig. 6). In both mammary gland tissues, a few spots with 1–3 cells contained cytoplasmic granular fluorescence (Fig. 6). The fluorescing cells were either part of the epithelial lining or were found free in the lactiferous lobules.
Caprine Mammary gland. Detection of C. burnetii bacteria (green) within two epithelial cells in a lactiferous lobule by fluorescence in situ hybridization (FISH). Four different fluorescein labeled oligonucleotide probes specifically targeting 16 S ribosomal RNA of C. burnetii were used. Bar = 20 μm
Discussion
In the present study, C. burnetii was diagnosed as the abortifacient pathogen in a dairy goat herd, and a vaccination program was implemented to control the disease and prevent excretion of the pathogen. Nevertheless, individually vaccinated goats shed C. burnetii sporadically over a period of approximately two years, which might have resulted in positive BTM samples, and this is in line with previous studies (Bauer et al. 2022; Boarbi et al. 2014; van den Brom et al. 2013). Therefore, vaccination of C. burnetii infected goats may not completely prevent C. burnetii excretion in milk, but may reduce it (Hogerwerf et al. 2011). In previous experimental studies, C. burnetii DNA was detected in mammary gland tissue from both udder halves and in the supramammary lymph nodes (Roest et al. 2012, 2020; Sánchez et al. 2006). In natural C. burnetii infected and vaccinated dairy goats, C. burnetii DNA was also identified in mammary gland tissue, but their supramammary lymph nodes tested negative, which is consistent with our findings. Viable C. burnetii microorganisms have not yet been detected in mammary gland tissue from ruminants. Although, C. burnetii showed in vitro a high replication rate in epithelial cells from bovine udder compared to epithelial cells from lung and placenta (Sobotta et al. 2017). The tropism of C. burnetii to mammary gland tissue seems to provide the basis for excretion in milk. Infection of the mammary gland appears to occur regardless of the pregnancy status of the goat (Roest et al. 2012, 2020). Moreover, the risk of C. burnetii transmission among goats through milk during milking activity remains uncertain. An experimental infection of dairy cows through the teat canal resulted in a five-day bacteremia and C. burnetii milk shedding for 63 days (Williams 1991). In contrast, dipping cattle teats into C. burnetii-contaminated milk for 10–19 weeks failed to establish an infection (Williams 1991). Taken together, the reasons and associated risks of chronic milk shedders in ruminants needs further targeted investigations.
An important question that arose in our study is why C. burnetii colonizes the udder in addition to its tropism for trophoblasts. For example, similar low/reduced oxygen levels in trophoblasts and the udder may play a role for the bacterial tissue tropism. So one factor might be the oxygen content in the udder tissue. Indeed, in goats, O2 uptake in the udder increases during late gestation and peaks during early lactation (Davis et al. 1979). The increasing oxygen consumption is thought to lead to localized chronic hypoxia (Zhao 2014). Hypoxia in mammary gland tissue and the accumulation of hypoxia-inducible factor (HIF)-1α could impair bacterial clearance and allow recurrent or chronic C. burnetii manifestation to occur (Hayek et al. 2022). Hypoxic conditions are found in inflamed and infected tissues (Jantsch and Schödel 2015), and chronic subclinical mastitis increases the likelihood of C. burnetii shedding in milk in cattle (Barlow et al. 2008). Our hypothesis is supported by the detection of C. burnetii in arteriosclerotic plaques in humans, which are also considered hypoxic (Hagenaars et al. 2014; Jantsch and Schödel 2015). Furthermore, C. burnetii was successfully cultivated from a diseased human heart valve under hypoxic conditions (Boden et al. 2015). Finally, the conditions that favor the colonization of C. burnetii in body tissues, including the mammary gland, need more elucidation in the future.
No specific pathogens were detected in routine bacteriological milk examination. Nevertheless, a mild, predominantly interstitial lymphoplasmacytic inflammation was found histopathologically in the mammary gland. Extensive lymphocytic infiltration was observed in the mammary gland from one goat after experimental infection with C. burnetii (Sánchez et al. 2006). In addition, CAEV also causes interstitial lymphocytic inflammation in the goat udder (Zink and Johnson 1994), but antibodies against CAEV were not detected in the goat by ELISA. Moreover, focal granulomatous inflammation was found in the mammary gland tissue of goat #67455. Granulomatous inflammation can be caused by various infectious and non-infectious reasons (Shah et al. 2017). In the present case granulomatous inflammation was always localized around corpora amylacea and was most likely interpreted as a foreign body reaction. Infectious causes of granuloma formation in goat udders are for example, Mycobacterium caprae and yeasts, and affected goats show severe clinical signs of mastitis (Ahmed et al. 2020; Singh et al. 1994), which were not present in the current case. Acute C. burnetii infection also leads to the formation of granulomas in infected human organs, and a lipid vacuole forms the center of these C. burnetii-specific granulomas (Raoult et al. 2005). However, granulomas have rarely been observed in chronic Q fever patients, and it is suggested that the absence of typical granulomas is due to the lack of a T-cell immune response (Maurin and Raoult 1999). Finally, C. burnetii did not induce the upregulation of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α, in bovine udder epithelia (Sobotta et al. 2017). Therefore, the histopathological findings revealed in goat #67455 can not be related to the presence of C. burnetii. The minor clinical impact of C. burnetii in the mammary gland is supported by the SCC value (< 300 × 103 cells/ml) of the bilateral milk specimens on the day of euthanasia. There is no legal limit for SCC in goats in the European Union. The SCC value varies widely between 270 and 2,000 × 103 cells/ml in goats without intramammary infection and also depends on physiological factors (Haenlein 2002; Paape et al. 2001). Therefore, we assume that the C. burnetii infection had no impact on the SCC of the present goat. In contrast, the SCC in cattle seems to be negatively influenced by C. burnetii (Barlow et al. 2008).
The BTM samples remain C. burnetii DNA negative after the removal of goat #67455. This does not rule out the excretion of the pathogen by others goats, as shown in Fig. 2. However, goat #67455 excreted the largest amount of C. burnetii DNA during sampling in November 2020 and March 2021, and BTM also tested positive on the same sample dates (Fig. 1). In contrast, no or only a small amount of C. burnetii DNA was detected in individual milk samples from goat #67455 (January and July 2020), but the BTM tested negative on the same dates. Therefore, the detection of C. burnetii in BTM appears to depend on the amount of pathogen excreted and the dilution effect of BTM. Studies comparing C. burnetii outcomes among individual milk shedders and BTM are rare. In dairy cows, a sensitivity of 82% (95% CI 69–95%) and a specificity of 70% (95% CI 59–81%) were estimated for BTM samples (Muskens et al. 2011). Similar results were reported by van den Brom and colleagues (2012) who evaluated a PCR assay for the detection of C. burnetii in BTM samples from goats. Finally, a single BTM sample can give a false-negative result, therefore repeated testing is required to confirm herd status.
Nowadays, BTM samples are commonly used to identify dairy goat herds infected with C. burnetii (Jansen et al. 2021; Jodełko et al. 2021; Khalili et al. 2015). Viable C. burnetii has been detected in raw milk from cattle (Loftis et al. 2010) and in raw milk cheese from sheep for up to 8 months (Barandika et al. 2019). This poses a risk for humans to become infected with Q fever through consumption of raw milk, which is becoming increasingly popular. Although seroconversion does occur after consumption of contaminated raw milk, acute Q fever cases are rare and appear to depend on the amount of milk consumed (Signs et al. 2012). Recent data suggest that the pasteurization temperature, according to the Codex Alimentarius, can be lowered by 1–2˚C to inactivate C. burnetii in raw milk, and this should be applied to prevent alimentary transmission (Wittwer et al. 2022).
The authors are aware of the limitation of the study. Only one goat was studied, but this is the first time that the presence of C. burnetii antigen and bacterial cells in mammary gland tissue has been demonstrated with IHC and FISH, respectively. Mammary gland tissue manifestation was not achieved by experimental conditions. Therefore, the findings from our field study are a further contribution to confirming the occurrence of chronic C. burnetii excretion in milk in dairy animals, although the reason for the potential manifestation of C. burnetii in the mammary gland tissue remains uncertain. Furthermore, a hypothesis on the possible influence of hypoxia in the udder on Coxiella colonization/replication has been proposed, which requires more intensive investigations under controlled conditions.
This field study demonstrates for the first time the presence of C. burnetii antigen and bacterial cells in mammary gland tissue from a naturally infected dairy goat using IHC and FISH, respectively. Moreover, the examined goat shed C. burnetii through milk on three out of four sampling dates and during a four-week examination period, which might be caused by the presence of C. burnetii in the mammary gland tissue. Persistent C. burnetii shedders should be removed from the herd to minimize the risk of C. burnetii transmission among animals, although the role of contaminated milk as an infectious source remains unclear. The growing popularity of purchasing raw milk directly from farms and consuming it without prior heating (EFSA Panel on Biological Hazards 2015; Sobotta et al. 2022) might pose a risk for acquiring Q fever. Although the digestive route might not pose a major public health threat, it could potentially play a role in the transmission of C. burnetii (Eldin et al. 2017). Therefore, heat treatment of raw milk from C. burnetii-infected herds is recommended (BfR 2010). This recommendation should also be applied to milk from goats that are both C. burnetii-positive and subsequently vaccinated, as vaccination appears unable to prevent C. burnetii shedding (Bauer et al. 2022).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed YF, Ezzo OH, Mansour S (2020) Some udder problems associated with productivity in goats. Egypt J Vet Sci 51:1–9. https://doi.org/10.21608/ejvs.2019.13869.1087
Álvarez-Alonso R, Basterretxea M, Barandika JF, Hurtado A, Idiazabal J, Jado I, Beraza X, Montes M, Liendo P, García-Pérez AL (2018) A Q fever outbreak with a high rate of abortions in a dairy goat farm: Coxiella burnetii shedding, environmental contamination and viability. Appl Environ Microbiol 84:e01650–e01618. https://doi.org/10.1128/aem.01650-18
Arricau-Bouvery N, Souriau A, Lechopier P, Rodolakis A (2003) Experimental Coxiella burnetii infection in pregnant goats: excretion routes. Vet Res 34:423–433. https://doi.org/10.1051/vetres:2003017
Arricau-Bouvery N, Souriau A, Bodier C, Dufour P, Rousset E, Rodolakis A (2005) Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine 23:4392–4402. https://doi.org/10.1016/j.vaccine.2005.04.010
Barandika JF, Alvarez-Alonso R, Jado I, Hurtado A, García-Pérez AL (2019) Viable Coxiella burnetii in hard cheeses made with unpasteurized milk. Int J Food Microbiol 303:42–45. https://doi.org/10.1016/j.ijfoodmicro.2019.05.010
Barlow J, Rauch B, Welcome F, Kim SG, Dubovi E, Schukken Y (2008) Association between Coxiella burnetii shedding in milk and subclinical mastitis in dairy cattle. Vet Res 39:23. https://doi.org/10.1051/vetres:2007060
Bauer B, Prüfer L, Walter M, Ganter I, Frangoulidis D, Runge M, Ganter M (2020) Comparison of Coxiella burnetii excretion between sheep and goats naturally infected with one cattle-associated genotype. Pathogens 9:652. https://doi.org/10.3390/pathogens9080652
Bauer BU, Schoneberg C, Herms TL, Runge M, Ganter M (2022) Surveillance of Coxiella burnetii shedding in three naturally infected dairy goat herds after vaccination, focusing on bulk tank milk and dust swabs. Vet Sci 9:102. https://doi.org/10.3390/vetsci9030102
Baumgärtner W, Dettinger H, Schmeer N, Hoffmeister E (1988) Evaluation of different fixatives and treatments for immunohistochemical demonstration of Coxiella burnetti in paraffin-embedded tissues. J Clin Microbiol 26:2044–2047. https://doi.org/10.1128/jcm.26.10.2044-2047.1988
Benson W, Brock DW, Mather J (1963) Serologic analysis of a penitentiary group using raw milk from a Q fever infected herd. Public Health Rep 78:707–710
BfR (2010) Q fever: transmission of Coxiella burnetii through the consumption of foods of animal origin unlikely. https://www.bfr.bund.de/cm/349/q_fever_transmission_of_coxiella_burnetii_through_the_consumption_of_foods_of_animal_origin_unlikely.pdf. 13 August 2023
BMEL (2014) Recommendations of hygienic measures for ruminant husbandries (Bekanntmachung von Empfehlungen für hygienische Anforderungen an das Halten von Wiederkäuern). https://tsis.fli.de/GlobalTemp/202307221923436853.pdf. 22 July 2023
Boarbi S, Mori M, Rousset E, Sidi-Boumedine K, Van Esbroeck M, Fretin D (2014) Prevalence and molecular typing of Coxiella burnetii in bulk tank milk in belgian dairy goats, 2009–2013. Vet Microbiol 170:117–124. https://doi.org/10.1016/j.vetmic.2014.01.025
Boden K, Wolf K, Hermann B, Frangoulidis D (2015) First isolation of Coxiella burnetii from clinical material by cell-free medium (ACCM2). Eur J Clin Microbiol Infect Dis 34:1017–1022. https://doi.org/10.1007/s10096-015-2321-1
Buijs SB, Weehuizen JM, Jensen TK, Boye M, Hermans MH, Nooijen PT, Hoepelman AI, Bleeker-Rovers CP, Oosterheert JJ, Wever PC (2022) Fluorescence in situ hybridization for detecting Coxiella burnetii in tissue samples from chronic Q fever patients clin. Microbiol Infect 28:1502e1501. 1502.e1505
Celina SS, Cerný J (2022) Coxiella burnetii in ticks, livestock, pets and wildlife: a mini-review. Front Veterinary Sci 9. https://doi.org/10.3389/fvets.2022.1068129
Davis AJ, Fleet IR, Goode JA, Hamon MH, Walker FM, Peaker M (1979) Changes in mammary function at the onset of lactation in the goat: correlation with hormonal changes. J Physiol 288:33–44
De Cremoux R, Rousset E, Touratier A, Audusseau G, Nicollet P, Ribaud D, David V, Le Pape M (2012) Assessment of vaccination by a phase I coxiella burnetii-inactivated vaccine in goat herds in clinical Q fever situation. FEMS Immunol Med Microbiol 64:104–106. https://doi.org/10.1111/j.1574-695X.2011.00892.x
Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller P-J, Chicheportiche C, Nezri M, Poirier R (1992) Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 french cases. Am J Med 93:427–434. https://doi.org/10.1016/0002-9343(92)90173-9
EFSA Panel on Biological Hazards (2015) Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA J 13:3940. https://doi.org/10.2903/j.efsa.2015.3940
Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, Mege J-L, Maurin M, Raoult D (2017) From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 30:115–190. https://doi.org/10.1128/CMR.00045-16
Fishbein D, Raoult D (1992) A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg 47:35–40
Frangoulidis D, Meyer H, Kahlhofer C, Splettstoesser WD (2012) Real-time’ PCR based detection of Coxiella burnetii using conventional techniques. FEMS Immunol Med Microbiol 64:134–136. https://doi.org/10.1111/j.1574-695X.2011.00900.x
Frangoulidis D, Walter MC, Antwerpben M, Zimmermann P, Janowetz B, Alex M, Böttcher J, Henning K, Hilbert A, Ganter M, Runge M, Münsterkötter M, Splettstoesser WD, Hanczaruk M (2014) Molecular analysis of Coxiella burnetii in Germany reveals evolution of unique clonal clusters. Int J Med Microbiol 304:868–876. https://doi.org/10.1016/j.ijmm.2014.06.011
Gale P, Kelly L, Mearns R, Duggan J, Snary EL (2015) Q fever through consumption of unpasteurised milk and milk products - a risk profile and exposure assessment. J Appl Microbiol 118:1083–1095. https://doi.org/10.1111/jam.12778
Haenlein GFW (2002) Relationship of somatic cell counts in goat milk to mastitis and productivity. Small Rumin Res 45:163–178. https://doi.org/10.1016/S0921-4488(02)00097-4
Hagenaars JCJP, Koning OHJ, van den Haak RFF, Verhoeven BAN, Renders NHM, Hermans MHA, Wever PC, van Suylen RJ (2014) Histological characteristics of the abdominal aortic wall in patients with vascular chronic Q fever. Int J Exp Pathol 95:282–289. https://doi.org/10.1111/iep.12086
Hayek I, Szperlinski M, Lührmann A (2022) Coxiella burnetii affects HIF1α accumulation and HIF1α target gene expression. Front Cell Infect Microbiol 12:867689. https://doi.org/10.3389/fcimb.2022.867689
Hogerwerf L, van den Brom R, Roest HIJ, Bouma A, Vellema P, Pieterse M, Dercksen DP, Nielen M (2011) Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, the Netherlands. Emerg Infect Dis 17:379–386. https://doi.org/10.3201/eid1703.101157
Jansen W, Cargnel M, Boarbi S, Mertens I, Van Esbroeck M, Fretin D, Mori M (2021) Belgian bulk tank milk surveillance program reveals the impact of a continuous vaccination protocol for small ruminants against Coxiella burnetii. Transbound Emerg Dis 69:e141–e152. https://doi.org/10.1111/tbed.14273
Jantsch J, Schödel J (2015) Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology 220:305–314. https://doi.org/10.1016/j.imbio.2014.09.009
Jodełko A, Szymańska-Czerwińska M, Rola JG, Niemczuk K (2021) Molecular detection of Coxiella burnetii in small ruminants and genotyping of specimens collected from goats in Poland. BMC Vet Res 17:341. https://doi.org/10.1186/s12917-021-03051-0
Khalili M, Diali HG, Mirza HN, Mosavi SM (2015) Detection of Coxiella burnetii by PCR in bulk tank milk samples from dairy caprine herds in southeast of Iran. Asian Pac J Trop Dis 5:119–122. https://doi.org/10.1016/S2222-1808(14)60638-1
Loftis AD, Priestley RA, Massung RF (2010) Detection of Coxiella burnetii in commercially available raw milk from the United States. Foodborne Pathog Dis 7:1453–1456. https://doi.org/10.1089/fpd.2010.0579
Maurin M, Raoult D (1999) Q fever. Clin. Microbiol Rev 12:518–553. https://doi.org/10.1128/Cmr.12.4.518
Morroy G, Keijmel SP, Delsing CE, Bleijenberg G, Langendam M, Timen A, Bleeker-Rovers CP (2016) Fatigue following acute Q-fever: a systematic literature review. PLoS ONE 11:e0155884. https://doi.org/10.1371/journal.pone.0155884
Muskens J, van Engelen E, van Maanen C, Bartels C, Lam TJ (2011) Prevalence of Coxiella burnetii infection in dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. Vet Rec 168:79. https://doi.org/10.1136/vr.c6106
Paape MJ, Poutrel B, Contreras A, Marco JC, Capuco AV (2001) Milk somatic cells and lactation in small ruminants. J Dairy Sci 84:E237–E244. https://doi.org/10.3168/jds.S0022-0302(01)70223-8
Pexara A, Solomakos N, Govaris A (2018) Q fever and prevalence of Coxiella burnetii in milk. Trends Food Sci Technol 71:65–72. https://doi.org/10.1016/j.tifs.2017.11.004
Raoult D, Marrie T, Mege J (2005) Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226. https://doi.org/10.1016/s1473-3099(05)70052-9
Reedijk M, Van Leuken JPG, Van Der Hoek W (2013) Particulate matter strongly associated with human Q fever in the Netherlands: an ecological study. Epidemiol Infect 141:2623–2633. https://doi.org/10.1017/S0950268813000460
Roest HJ, van Gelderen B, Dinkla A, Frangoulidis D, van Zijderveld F, Rebel J, van Keulen L (2012) Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLoS ONE 7:e48949. https://doi.org/10.1371/journal.pone.0048949
Roest HIJ, Dinkla A, Koets AP, Post J, van Keulen L (2020) Experimental Coxiella burnetii infection in non-pregnant goats and the effect of breeding. Vet Res 51:74. https://doi.org/10.1186/s13567-020-00797-7
Sánchez J, Souriau A, Buendía AJ, Arricau-Bouvery N, Martínez CM, Salinas J, Rodolakis A, Navarro JA (2006) Experimental Coxiella burnetii infection in pregnant goats: a histopathological and immunohistochemical study. J Comp Pathol 135:108–115. https://doi.org/10.1016/j.jcpa.2006.06.003
Shah KK, Pritt BS, Alexander MP (2017) Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 7:1–12. https://doi.org/10.1016/j.jctube.2017.02.001
Signs KA, Stobierski MG, Gandhi TN (2012) Q fever cluster among raw milk drinkers in Michigan, 2011. Clin Infect Dis 55:1387–1389. https://doi.org/10.1093/cid/cis690
Singh M, Gupta PP, Rana JS, Jand SK (1994) Clinico-pathological studies on experimental cryptococcal mastitis in goats. Mycopathologia 126:147–155. https://doi.org/10.1007/BF01103768
Sobotta K, Bonkowski K, Liebler-Tenorio E, Germon P, Rainard P, Hambruch N, Pfarrer C, Jacobsen ID, Menge C (2017) Permissiveness of bovine epithelial cells from lung, intestine, placenta and udder for infection with Coxiella burnetii. Vet Res 48:23. https://doi.org/10.1186/s13567-017-0430-9
Sobotta K, Bonkowski K, Heydel C, Henning K, Menge C (2022) Phenotype of Coxiella burnetii strains of different sources and genotypes in bovine mammary gland epithelial cells. Pathogens 11:1422. https://doi.org/10.3390/pathogens11121422
Ullah Q, Jamil T, Saqib M, Iqbal M, Neubauer H (2022) Q Fever-A neglected zoonosis. Microorganisms 10:1530. https://doi.org/10.3390/microorganisms10081530
van den Brom R, van Engelen E, Luttikholt S, Moll L, van Maanen K, Vellema P (2012) Coxiella burnetii in bulk tank milk samples from dairy goat and dairy sheep farms in the Netherlands in 2008. Vet Rec 170:310. https://doi.org/10.1136/vr.100304
van den Brom R, van Engelen E, Vos J, Luttikholt SJM, Moll L, Roest HIJ, van der Heijden HMJF, Vellema P (2013) Detection of Coxiella burnetii in the bulk tank milk from a farm with vaccinated goats, by using a specific PCR technique. Small Rumin Res 110:150–154. https://doi.org/10.1016/j.smallrumres.2012.11.024
van Roeden SE, Holsboer EW, Oosterheert JJ, van Kats JP, van Beckhoven J, Hogema BM, van Wijk MJ (2018) Seroprevalence of Coxiella burnetii antibodies and chronic Q fever among post-mortal and living donors of tissues and cells from 2010 to 2015 in the Netherlands. Euro Surveill 23:17–00384. https://doi.org/10.2807/1560-7917.ES.2018.23.9.17-00384
Vellema P, Santman-Berends I, Dijkstra F, van Engelen E, Aalberts M, ter, Bogt-Kappert C, van den Brom R (2021) Dairy sheep played a minor role in the 2005–2010 human Q Fever outbreak in The Netherlands compared to dairy goats. Pathogens 10, 1579. https://doi.org/10.3390/pathogens10121579
Williams JC (1991) Infectivity, virulence, and pathogenicity of Coxiella burnetii for various hosts. In: Williams JC, Thompson HA (eds) Q fever: the Biology of Coxiella burnetii. CRC Press, Boca Raton, pp 21–72
Wittwer M, Hammer P, Runge M, Valentin-Weigand P, Neubauer H, Henning K, Mertens-Scholz K (2022) Inactivation kinetics of Coxiella burnetii during high-temperature short-time pasteurization of milk. Front Microbiol 12:3975. https://doi.org/10.3389/fmicb.2021.753871
Wolf-Jäckel GA, Strube ML, Schou KK, Schnee C, Agerholm JS, Jensen TK (2021) Bovine abortions revisited—enhancing abortion diagnostics by 16S rDNA amplicon sequencing and fluorescence in situ hybridization. Front Vet Sci 8:623666. https://doi.org/10.3389/fvets.2021.623666
Zhao FQ (2014) Biology of glucose transport in the mammary gland. J Mammary Gland Biol Neoplasia 19:3–17. https://doi.org/10.1007/s10911-013-9310-8
Zink MC, Johnson LK (1994) Pathobiology of lentivirus infections of sheep and goats. Virus Res 32:139–154. https://doi.org/10.1016/0168-1702(94)90039-6
Acknowledgements
Special thanks go to the goat farmer who supported the study. We would like to thank Annika Wolf for her excellent technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Federal Ministry of Education and Research (BMBF) under project numbers 01Kl1726B and 01KI2008B as part of the Research Network Zoonotic Infectious Diseases.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Benjamin U. Bauer: Conceptualization, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization; Martin Peters: Validation, Investigation, Resources, Writing – Review & Editing, Visualization; T. Louise Herms: Validation, Investigation, Writing – Review & Editing; Martin Runge: Validation, Writing – Review & Editing, Supervision, Project administration, Funding acquisition, Peter Wohlsein: Investigation, Resources, Writing – Review & Editing, Visualization; Tim K. Jensen: Investigation, Resources, Writing – Review & Editing, Visualization; Martin Ganter: Conceptualization, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The identification, removal and safe disposal of persistent C. burnetii shedders from a herd is in accordance with the “Recommendations of hygienic measures for ruminant husbandries” issued by the German Federal Ministry for Food and Agriculture (BMEL 2014). Therefore, this field investigation did not require official or institutional ethical approval because all samples were collected by routine diagnostic procedures to improve animal health. This is in accordance with German animal welfare legislation and the EU Directive 2010/63/EU for animal experiments. All animals were handled in accordance with high ethical standards and national legislation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, B.U., Peters, M., Herms, T.L. et al. Detection of Coxiella burnetii in the mammary gland of a dairy goat. Vet Res Commun (2024). https://doi.org/10.1007/s11259-023-10233-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-023-10233-8