Abstract
Nitric oxide (NO) was proposed to be an important molecule against some microorganisms. In this study, we investigated the inhibitory effect of NO on the infection by porcine reproductive and respiratory syndrome virus (PRRSV) in vitro and the role of NO in the defense against PRRSV. Our results indicated that exogenous NO did not inhibit PRRSV infection. Unexpectedly, N-acetylpenicillamine (NAP), a commonly used compound as negative control for NO-producing reagents, inhibited PRRSV replication. Thus, the inhibition effect of NAP on PRRSV replication was further explored. We found that the maximal inhibition effect of NAP on PRRSV replication was achieved upon treatment 1 h after virus infection and the virus yield was reduced by approximately 50 fold in the presence of 400 μM NAP. An obvious inhibitory effect on viral RNA and protein synthesis was also observed. However, the inhibitory effect was only achieved at early phase of virus infection. The normal virus yield could be restored upon the removal of NAP treatment. The inhibitory effect might be caused by sulfhydryl-reducing capacity and metal chelating properties of NAP. These studies suggested that (i) NO production or NO synthase (NOS) expression profiling may not be a reliable index for the immune response to PRRSV; (ii) NAP could inhibit the replication of PRRSV.
Similar content being viewed by others
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is one of the most important diseases of swine. It is characterized by severe reproductive failure in sows, and respiratory distress in piglets and growing pigs (Pejsak and Markowska-Daniel 1997). The PRRS virus (PRRSV), the causative agent, is a positive-strand RNA virus belonging to the family Arteriviridae (Meulenberg 2000). This family also includes murine lactate dehydrogenase-elevating virus (LDV), equine arteritis virus (EAV) and simian hemorrhagic fever virus (SHFV) (Meulenberg 2000). Currently, PRRSV isolates can be divided into two distinct groups, which are represented by Lelystad virus in Europe and VR-2332 in the United States, respectively (Wensvoort et al. 1991; Benfield et al. 1992). The viral genome contains nine open reading frames (ORFs): ORF1a, ORF1b, ORF2a, ORF2b and ORFs3-7 (Wu et al. 2001).
Nitric oxide (NO) is a highly reactive molecule generated by nitric oxide synthase (NOS), which converts L-arginine to citrulline and NO (Moncada et al. 1991). The NO molecule mediates numerous physiological functions as a vasodilator, neurotransmitter, immune regulator, antimicrobial and antiviral agent (Bogdan 2001; Cherayil and Antos 2001; Reiss and Komatsu 1998). In addition, NO plays an important role in the defense against herpes simplex virus type 1, vesicular stomatitis virus, Japanese encephalitis virus, murine hepatitis virus, vaccinia virus, influenza virus, and severe acute respiratory syndrome coronavirus (Åkerström et al. 2005; Bi and Reiss 1995; Harris et al. 1995; Lin et al. 1997; Pope et al. 1998; Rimmelzwaan et al. 1999). Moreover, NO also participates in immune responses and possesses inhibitory properties against various pathogens (Harris et al. 1995; Lin et al. 1997; Pope et al. 1998).
N-acetylpenicillamine (NAP) is the acetylated form of D-penicillamine (DPA). In some experiments, due to the absence of NO-donating S-nitro group, NAP was used as the negative control for NO donor SNAP. DPA is a potent metal chelator and belongs to the amino acids containing a thiol group. DPA can be used as antidotes for toxic metals, such as mercury poisoning. NAP, the acetylated DPA, retains these properties. To date, NAP is only used as the negative control for SNAP to study the inhibitory effect of NO on virus replication in previous studies, and its antiviral activity was not reported.
In the present study, we first investigated the potential inhibitory effect of NO on PRRSV replication in Marc-145 cells. Although exogenous NO was released by NO donor, SNAP, in Marc-145 cells, the replication of PRRSV remained unaffected. Unexpectedly, NAP exhibited an inhibitory effect on PRRSV replication. Therefore, this intriguing inhibitory effect of NAP on PRRSV replication was further investigated.
Materials and methods
Virus and cells
PRRSV strain YA1 used in this study was isolated from lungs of field pigs at the acute stage of PRRSV infection in China in 2001 and later identified as a highly virulent North American type isolate. The PRRSV was propagated and titered in Marc-145 cells.
Reagents
SNAP (a NO-producing compound), NAP (a negative control for SNAP due to the absence of NO-donating S-nitroso group) and DPA (an analog of NAP without N-acetylation) were purchased from Sigma (USA). The solutions were prepared by dissolving 5 mM SNAP, NAP and DPA into dimethyl sulfoxide-H2O with a ratio of 10:47 in volume and stored at −70 °C for the future experiments.
Measurement of NO concentration
The amount of NO produced in medium was determined by measuring its stable form, NO -2 (Lin et al. 1997). The sample was added with equal volume of Greiss reagent (1% sulfanilamide, 0.1% N-1-naphthylethy-lenediamine and 5% H3PO4) (Sigma, USA) and incubated in 96-well microtiter plates at room temperature for 10 min. The color development was measured at the wavelength of 540 nm with an automated microplate reader (Model EL309; Bio-Tek, Inc, USA).
MTT assay for cytotoxicity
Cell cytotoxicity was determined by standard MTT assay with appropriate concentrations of different reagents (Lin et al. 1997).
Virus infection and titer determination
In order to infect cells with PRRSV, Marc-145 cells in 24-well plates were first incubated with PRRSV at a multiplicity of infection (MOI) of 0.05 at 37 °C for 1 h. Then, unbound viruses were removed by washing with PBS (pH 7.4) for three times, and fresh medium was added to each plate for further incubation at 37 °C. During experiments, the culture medium was supplemented with 100, 200 and 400 μM SNAP, NAP or DPA. The medium containing the same concentrations of agents were replenished every 6–8 h during the culture period. As a negative control, the solvent alone (dimethyl sulfoxide-H2O) was added to the culture medium. Infectious PRRSV in the supernatant was quantitatively evaluated by plaque-forming assay in Marc-145 cells. Various virus dilutions were added to the monolayer of Marc-145 cells and incubated at 37 °C for 1 h. After the incubation, the cells were washed with PBS (pH 7.4) and overlaid with 1% methyl cellulose containing DMEM F-12 medium plus 2% FBS. After an incubation of 4 days, the cells were stained with 0.5% crystal violet.
Semi-quantitative RT-PCR
Total RNA was extracted from Marc-145 cells with TRIzol® reagent (Omega, USA) according to the manufacturer’s instruction. RT-PCR was performed with One-Step RNA PCR kit (AMV) (TaKaRa, Japan). The primer pair for PRRSV ORF7 was as follows: P7-1, 5'-CCCCTAGTGAGCGGCAATT-3'; and P7-2, 5'-AGTCCCAGCGCCTTGATTAA-3'. The housekeeping gene, β-actin gene, was used as a constitutively-expressed control. Its primers were as follows: pβ-actinF, 5'-ACTGTGCCCATCTACGAG-3'; and pβ-actinR, 5'-GTTGCGTTACACCCTTTC-3'. The 430-bp ORF7 fragments were visualized and photographed under UV illumination.
Western blot analysis
The cells were washed twice with PBS (pH 7.4) and lysed in lysis buffer (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]). Solubilized proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins in the gel were transferred to a nitrocellulose membrane and probed with rabbit hyperimmune sera against N protein followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Southern Biotechnology). The housekeeping gene, β-actin gene, was used as a constitutively-expressed control in Western blot. Blots were developed by the enhanced chemiluminescence Western blot detection system (Amersham) and directly exposed to image in Molecular Image Station 2000MM (Kodak).
Indirect immunofluorescence analysis
After the treatment with/without chemicals and PRRSV infection, the infected cells were detected with rabbit anti-PRRSV N protein polyclonal antibodies as described previously (Tian et al., 2009). The labeled cells were examined by fluorescence microscope (Olympus IX70, Japan).
Results
Effects of SNAP and NAP on PRRSV infection
In order to investigate NO release in Marc-145 cells after SNAP treatment, the cells were treated with different concentrations of SNAP and NAP or culture medium over a period of 12 h. Cell culture supernatants were collected and NO production were measured. After SNAP treatments, the release of NO in medium was observed in a concentration-dependent manner, but neither NAP nor culture medium released a detectable amount of NO. All drug treatments did not exhibit any negative impact on cell growth by MTT assay.
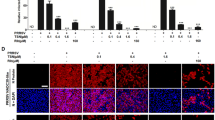
Compared with untreated cells, SNAP could not inhibit PRRSV replication in Marc-145 cells based on the NO release. Unexpectedly, NAP, the “commonly used” negative control for NO-donor SNAP had a significant antiviral activity against PRRSV replication (Fig. 1a) at low infectious dose (MOI = 0.05). The inhibitory effect was observed from 6 to 105 h post-infection. At 35 h post-infection, virus titer was decreased from 1260 to 190, 110 and 50 pfu/1 ml under 100, 200 and 400 μM NAP treatments, respectively. However, the inhibition effect was lost at higher infectious doses (MOI = 0.1 and 0.5) (Fig. 2). In order to investigate the inhibitory kinetics of NAP on PRRSV replication and its inhibitory mechanisms, the optimal inhibition conditions were also investigated. In our experiments, inhibition analysis of NAP on PRRSV replication was carried out by 400 μM NAP at low infectious dose (MOI = 0.05).
Kinetics of PRRSV production in infected cells with NAP treatments. a Effect of SNAP and NAP on PRRSV replication in Marc-145 cells. PRRSV-infected cells (MOI = 0.05) were treated with SNAP or NAP at the concentrations of 0, 100, 200 and 400 μM. Cell supernatant was harvested and virus titers were determined by phage-forming assay. b Effect of NAP on PRRSV replication in host cells. The cells were incubated with NAP for 2 h prior to infection. c Comparative analysis for inhibition effect of NAP and DPA on PRRSV replication
Kinetics of PRRSV production in infected cells with NAP treatments
The inhibitory effect of NAP in Marc-145 cells was investigated by determining the kinetics of PRRSV production under different NAP treatments. Approximately 50-fold decrease of virus yield in PRRSV-infected cells was observed in the presence of 400 μM NAP, as shown in Fig. 1a. At the early stage of experiments, NAP could completely inhibit the detectable propagation of PRRSV. The inhibitory effect on viral release was observed in a concentration-dependent manner of NAP. However, it lost at higher infectious doses (MOI = 0.1 and 0.5) (Fig. 2). In order to investigate inhibitory kinetics of NAP on PRRSV and inhibition mechanisms, the optimal condition was also investigated in following tests. The analysis of NAP inhibitor to PRRSV was conducted using 400 μM NAP at a low infectious dose (MOI = 0.05). The inhibitory kinetics of NAP to PRRSV was stable and easily observed which provided sufficient conditions for analyzing inhibitory mechanisms. Meanwhile, to better understand the inhibition mechanism of NAP, the inhibition of DPA, an analog of NAP without N-acetylation, was also investigated.
When the cells were incubated with NAP for 2 h prior to the infection, total virus yield remained unaffected (Fig. 1b), indicating that NAP had no effect on the capability of host cells to support subsequent viral replication. Compared with the inhibitory effect of NAP, PRRSV remained unaffected by DPA (Fig. 1c).
Effect of NAP on PRRSV RNA synthesis
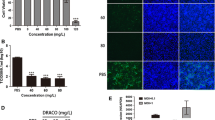
In order to investigate whether NAP treatments reduce the amount of viral RNA in infected cells, total RNA was extracted from infected Marc-145 cells with or without NAP treatment. As shown in Fig. 3a, the amount of viral RNA was significantly reduced at 36 h post-infection, indicating that NAP could decrease viral RNA in a dose-dependent manner.
Effect of NAP on PRRSV specific RNA and protein synthesis. Marc-145 cells infected with PRRSV were treated by NAP at the concentration of 0, 100, 200 and 400 μM. ORF7 of PRRSV was detected by RT-PCR to evaluate the amount of viral RNA. Beta-actin was detected as a housekeeping gene. N protein of PRRSV was detected by Western blot and IFA using polyclonal antibody against N protein. a The product of RT-PCR was photographed under UV illumination and analyzed by gel layer scanning. b The expression level of N protein was analyzed by Western blot. c–e N protein was evaluated by indirect immunofluorescence analysis in PRRSV-infected Marc-145 cells without treatments (c), PRRSV-infected Marc-145 cells with NAP treatment (d) and mock-infected cells (e). Magnification, 200×
Effect of NAP on PRRSV protein synthesis
Since NAP could reduce the yield of viral RNA, a significant inhibitory effect on viral protein synthesis should be observed. At 36 h post-infection, N protein expression level of PRRSV was examined by Western blot with a polyclonal antibody against N protein (Fig. 3b). Beta-actin expression was used as the loading control. In addition, as shown by immunofluorescence analysis (IFA) (Fig. 3c–e), compared with PRRSV-infected cells without treatment, NAP treatments on PRRSV-infected cells resulted in an obvious decline in the number or intensity of stained cells against N protein.
Effector phase and reversibility of NAP inhibition
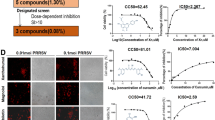
NAP inhibition on PRRSV replication at the early and late phases of virus infection as well as its reversibility was also studied. At the inhibitory phase, NAP was added to infected cells at 1, 3 and 12 h post-infection. The viral yield was significantly reduced at 1 and 3 h post-infection (Fig. 4a). When NAP was added to infected cells at 1 h post-infection, virus titer was detected until 21 h post-infection. In contrast, at 12 h post-infection for adding NAP, the viral yield did not exhibit significant difference between treated and untreated-cells, which indicated that NAP inhibitory effect was abolished at this time point, and inhibitory effect of NAP could be achieved only at early phase of virus infection.
The analysis of effector phase and reversibility of NAP inhibition. a Effector phase of NAP inhibition was investigated by adding NAP to infected cells at different time points post-infection. b Reversibility of NAP inhibition was detected. NAP was added to infected cells at 1 h post-infection and the NAP treatment was removed after 2 h by washing the cells. The cell culture was replenished in fresh medium without NAP. Virus titers were detected to compare infected cells with and without treatments at different time points post-infection
To determine the inhibition reversibility, NAP was added to the infected cells at 1 h post-infection and the treatment was removed after 2 h by washing the treated cells. The cell culture was then replenished in fresh medium without NAP treatment. The results showed that virus titers from NAP treated cells followed by washing treatment were similar to those of the controls (Fig. 4b). Therefore, the inhibitory effect was reversible.
Discussion
In this study, SNAP treatment was used to induce exogenous NO, but its inhibitory effect on PRRSV infection was not observed. Unexpectedly, NAP, originally used as the negative control of SNAP, exhibited an inhibitory effect on PRRSV replication in a concentration-dependent manner. NAP treatment could inhibit both PRRSV viral RNA and protein synthesis and its inhibitory effect was achieved at 1 to 3 h after virus infection. Moreover, the normal virus yield could be restored after the removal of NAP treatment.
NO was reported to be a crucial effector molecule against microorganism infection (Nathan and Xie 1994). In addition, NO could modulate the gene expression of multiple signal transduction pathways (Huang et al. 1998). Therefore, NO production or NOS expression profiling was often considered as an important index of immune responses against some pathogens (Gamba et al. 2004). However, previous experimental evidences indicated that NO activity varied among different cells or tissues and NO formation could not be detected in porcine immune cells. In addition, NO level was not changed in lung macrophages of the PRRSV-infected pigs (Boissel et al. 1998). Recently, the reports also confirmed that NO was not able to significantly inhibit PRRSV replication in vivo (Jung et al. 2010). Exogenous or endogenous NO and inducible NOS expression exhibited no apparent antiviral effect on Sendai virus (Z strain) and influenza A virus (H2N2) in MDCK cells (Yoshitake et al. 2004). Similarly, Our results demonstrated that sufficient NO production could not suppress the propagation of PRRSV in vitro. Based on these results, it is reasonable to propose that NO production or NOS expression profiling may not be a key index for the immune response to PRRSV.
Unexpectedly, we found that NAP, a structural analog of cysteine and a metal chelator, could inhibit the replication of PRRSV in Marc-145 cells. Due to NAP treatment, a significant reduction of viral yield and a remarkable inhibition of both viral RNA and protein synthesis were observed. However, the inhibitory activity was not attributed to the involvement of Marc-145 cells. NAP could result in the maximal inhibitory effect on the replication of PRRSV at the 1 h post-infection and total abolishment of PRRSV replication at 12 h post-infection. This observation strongly suggested that NAP only interrupted PRRSV replication during the early phase of virus infection. The inhibitory effect was reversible, and the normal virus yield could be recovered after the removal of the drug. In addition, DPA, an analog of NAP with the free amino group, could not inhibit PRRSV replication (Fig. 1c). The results indicated that acetylation may play an important role on PRRSV replication.
Previous studies demonstrated that DPA could prevent trans-activation of human immunodeficiency virus type-1 (HIV-1) LTR (Chandra et al., 1988). A docking study further revealed a selective binding of DPA to the cysteine-rich region of TAT protein in HIV (Kanyalkar et al. 2002). Here we found that the inhibitory activity of NAP and the inactivity of DPA on PRRSV replication. These results enriched our understanding of NAP and DPA, although the exact mechanisms of inhibition by NAP still need to be further studied.
The inhibitory effect may result from the disruption of disulfide bonds by the free thiol group of NAP. Because the thiol group of SNAP is nitrosated, SNAP exhibits no disulfide reducing activity (Fig. 5). Disulfide bonds play an important role in assembling and stabilizing viral capsid structure of several viruses with icosahedral nucleocapsids (Jeng et al. 1991; Li et al. 2002; Wootton and Yoo 2003). Human cytomegalovirus and vesicular stomatitis virus (VSV) are also demonstrated to be vulnerable to sulfhydryl reagents (Baum et al. 1996; Beatrice and Wagner 1980). Alternatively, the inhibitory effect of NAP on PRRSV replication could be related to its metal chelating properties. It was reported that the replication of human immunodeficiency virus (HIV), hepatitis C virus (HCV) and flavivirus can be inhibited by different chelating agents (Chyan-Jang et al. 2008; Koch et al. 2006; Van-Asbeck et al. 2000).The exact inhibitory mechanisms of PRRSV replication need to be verified.
In summary, our studies provide two crucial evidences. First, NO have no effect on PRRSV replication in Marc-145 cells. Second, NAP can inhibit the replication of PRRSV. These findings could contribute to the development of effective anti-PRRSV agents.
References
Åkerström S, Mousavi-Jazi M, Klingström J, Leijon M, Lundkvist Å, Mirazimi A (2005) Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 79: 1966–1969.
Baum EZ, Siegel MM, Bebernitz GA, Hulmes JD, Sridharan L, K-Tabei LS, Johnston SH, Wildey MJ, Nygaard J, Jones TR, Gluzman Y (1996) Inhibition of human cytomegalovirus UL80 protease by specific intramolecular disulfide bond formation. Biochemistry 35: 5838–5846.
Beatrice ST, Wagner RR (1980) Effect of sulfhydryl reagents on the infectivity of vesicular stomatitis virus. Virology 100: 246–251.
Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D (1992) Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest 4: 127–133.
Bi Z, Reiss CS (1995) Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol 69: 2208–2213.
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2: 2907–2916.
Boissel JP, Schwarz PM, Förstermann U (1998) Neuronal-type NO synthase: transcript diversity and expressional regulation. Nitric Oxide 2: 337–349.
Chandra A, Demirhan I, Arya SK, Chandra P (1988) D-penicillamine inhibits transactivation of human immunodeficiency virus type-1 (HIV-1) LTR by transactivtor protein. FEBS Lett. 236: 282–286.
Cherayil BJ, Antos D (2001) Inducible nitric oxide synthase and Salmonella infection. Microbes Infect 3: 771–776.
Chyan-Jang L, Hui-Ru L, Ching-Len L, Yi-Ling L (2008) Cholesterol effectively blocks entry of flavivirus. J Virol 82: 6470–6480.
Gamba G, Cavalier H, Courreges MC, Massouh EJ, Benencia F (2004) Early inhibition of nitric oxide production increases HSV-1 intranasal infection. J Med Virol 73: 313–322.
Harris N, Buller RML, Karupiah G (1995) Gamma interferon-induced nitric oxide-mediated inhibition of vaccinia virus replication. J Virol 69: 910–915.
Huang FP, Niedbala W, Wei XQ, Xu D, Feng GJ, Robinson JH, Lam C, Liew FY (1998) Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol 28: 4062–4070.
Jeng KS, Hu CP, Chang CM (1991)Differential formation of disulfide linkages in the core antigen of extracellular and intracellular hepatitis B virus core particles. J Virol 65: 3924–3927.
Jung K, Gurnani A, Renukaradhya GJ, Saif LJ (2010) Nitric Oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol doi:10.1016/j.vetimm.2010.03.022.
Kanyalkar DM, Chandra A, Doerr HW, Coutinho E, Loewer J, Saran LA, Chandra P (2002) Docking studies reveal a selective binding of D-penicillamine to the transactivator protein of human immunodeficiency virus type 1. FEBS Lett 516: 43–46.
Koch U, Attenni B, Malancona S, Colarusso S, Conte I, Di-Filippo M, Harper S, Pacini B, Giomini C, Thomas S, Incitti I, Tomei L, De-Francesco R, Altamura S, Matassa VG, Narjes F (2006) 2-(2-Thienyl)-5,6-dihydroxy-4-carboxypyrimidines as inhibitors of the hepatitis C virus NS5B polymerase: discovery, SAR, modeling, and mutagenesis. J Med Chem 49: 1693–1705.
Li PP, Nakanishi A, Clark SW, Kasamatsu H (2002) Formation of transitory intrachain and interchain disulfide bonds accompanies the folding and oligomerzation of simian virus 40 Vp1 in the cytoplasm. Proc Natl Acad Sci USA 99: 1353–1358.
Lin YL, Huang YL, Ma SH, Yeh CT, Chiou SY, Chen LK, Liao CL (1997) Inhibition of Japanese Encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol 71: 5227–5235.
Meulenberg JJ (2000) PRRSV, the Virus. Vet Res 31: 11–21.
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142.
Nathan C, Xie QW (1994) Nitric oxide synthase: roles, tolls, and controls. Cell 78: 915–918.
Pejsak Z, Markowska-Daniel I (1997) Losses due to porcine reproductive and respiratory syndrome in a large swine farm. Comp Immunol Microbiol Infect Dis 20:345–352.
Pope M, Marsden PA, Sloan S, Fung LS, Ning Q, Ding JW, Leibowitz JL, Phillips MJ, Levy GA (1998) Resistance to murine hepatitis virus strain 3 is dependent on production of nitric oxide. J Virol 72:7084–7090.
Reiss CS, Komatsu T (1998) Does nitric oxide play a critical role in viral infections? J Virol 72:4547–4551.
Rimmelzwaan GF, Baars MMJW, de-Lijster P, Fouchier RAM, Osterhaus AME (1999) Inhibition of influenza virus replication by nitric oxide. J Virol 73:8880–8883
Tian ZJ, An TQ, Zhou YJ, Peng JM, Hu SP, Wei TC, Jiang YF, Xiao Y, Tong GZ (2009) An attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) protects piglets against HP-PRRS. Vet Microbiol 138: 34–40.
Van-Asbeck BS, Georgiou VNA, van der-Bruggen T, Oudshoorn M, Nottet HS, Marx JJ (2000) Anti-HIV effect of iron chelators: different mechanisms involved. J Clin Virol 20: 141–147
Wensvoort G, Terpstra JM, Pol EA, tea-Laak M, Bloemraad EP, Kluyver C, Kragten C, van-Buiten L, den-Besten A, Wagenaar F (1991) Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet Q 13:121–130
Wootton SK, Yoo DW (2003) Homo-oligomerization of the porcine reproductive and respiratory syndrome virus nucleocapsid protein and the role of disulfide linkages. J Virol 77: 4546–4557.
Wu WH, Fang Y, Farwell R, Steffen-Bien M, Rowland RR, Christopher-Hennings J, Nelson A (2001) A 10 kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology 287: 183–191.
Yoshitake J, Akaike T, Akuta T, Tamura F, Ogura T, Esumi H, Maeda H (2004) Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity. J Virol 78: 8709–8719.
Acknowledgments
This work was supported by the National Basic Research Program (973) of China (2005CB523200), The National Key Technology R&D Program of China (2007BAD86B06), and the National Natural Sciences Foundation of China (30871871, 30800046).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Fang, L., Luo, R. et al. N-acetylpenicillamine inhibits the replication of porcine reproductive and respiratory syndrome virus in vitro. Vet Res Commun 34, 607–617 (2010). https://doi.org/10.1007/s11259-010-9435-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-010-9435-9