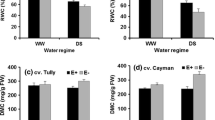
Abstract
Symbiotic associations with microbes can help plants to respond to environmental changes. In this study, we investigated how colonization by root endophytic fungi enhances performance of Chenopodium quinoa and its ability to cope with extended periods of drought. The fungus Penicillium minioluteum, which was isolated from quinoa naturally occurring in the Atacama Desert, was used for endophyte colonization. We developed a greenhouse experiment, subjecting endophyte-infected (E+) and endophyte-free (E−) plants to two treatments: water deficit and abundant water availability. Differences in plant performance, photosynthesis, water-use efficiency and photochemical efficiency between E+ and E− plants under both water treatments were examined. We assessed the nature of the plant–symbiont interaction (parasitic or mutualistic) under both treatment conditions. We found that P. minioluteum initially affected radicle growth, subsequently triggering improvements in root formation, with the latter particularly evident under drought conditions. Under low water, E+ plants demonstrated a 40% improvement in root formation relative to E− plants; however, physiological responses to drought were not demonstrably enhanced by the presence of endophytic fungi. The nature of the interaction appeared to be positive, but only under conditions of water stress. Our study demonstrates that, in C. quinoa, P. minioluteum assists in coping with water stress primarily by affecting substantial root biomass adjustments, and that host benefits in this relationship are conferred in conditions of stress only.




Similar content being viewed by others
References
Adams AE, Kazenel MR, Rudgers JA (2017) Does a foliar endophyte improve plant fitness under flooding? Plant Ecol 218:711–723
Alvarez-Flores R, Winkel T, Nguyen-Thi-Truc A, Joffre R (2014) Root foraging capacity depends on root system architecture and ontogeny in seedlings of three Andean Chenopodium species. Plant Soil 380:415–428
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Arnold AE, Engelbrecht BMJ (2007) Fungal endophytes double minimum leaf conductance in seedlings of a tropical tree. J Trop Ecol 23:369–372
Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA (2000) Are tropical fungal endophytes hyperdiverse? Ecol Lett 3:267–274
Bascuñán-Godoy L, Sanhueza C, Cuba M, Zuñiga GE, Corcuera LJ, Bravo L (2012) Cold-acclimation limits low temperature induced photoinhibition by promoting a higher photochemical quantum yield and a more effective PSII restoration in darkness in the Antarctic rather than the Andean ecotype of Colobanthus quitensis Kunt Bartl (Cariophyllaceae). BMC Plant Biol 12:114
Bascuñán-Godoy L, Reguera M, Blumwald YM, Blumwald E (2016) Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa willd. Planta 243:591–603
Bertero HD, Ruiz RA (2010) Reproductive partitioning in sea level quinoa (Chenopodium quinoa Willd.) cultivars. Field Crops Res 118:94–101
Bhargava A, Shukla S, Ohri D (2006) Chenopodium quinoa—an Indian perspective. Ind Crops Prod 23:73–87
Bray EA (2007) Plant responses to water deficit. Trends Plant Sci 2:48–54
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits—prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Cheplick GP (2009) Conceptual model for the analysis of plant-endophyte symbiosis in relation to abiotic stress. In: White JF, Torres MS (eds) Defensive mutualism in microbial symbiosis. CRC Press, Boca Raton, pp 322–323
Cheplick GP, Faeth SH (2009) Ecology and evolution of the grass-endophyte symbiosis. Oxford University Press, Oxford
Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:99–127
Contreras-Cornejo HA, Macías-Rodriguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxindependent mechanism in. Plant Physiol 149:1579–1592
Cusack D (1984) Quinoa: grain of the Incas. Ecologist 14:21–31
Dai C-C, Yu B-Y, Li X (2008) Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr J Biotechnol 7:3505–3510
Davitt AJ, Chen C, Rudgers JA (2011) Understanding context-dependency in plant–microbe symbiosis: the influence of abiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environ Exp Bot 71:137–145
De Micco V, Aronne G (2012) Morpho-anatomical traits for plant adaptation to drought. In: Aroca R (ed) Plant responses to drought stress: from morphological to molecular features. Springer, Heidelberg, pp 37–61
Elmi AA, West CP (1995) Endophyte effects on tall fescue stomatal response, osmotic adjustment and tiller survival. New Phytol 131:61–67
Fuentes F, Bhargava A (2011) Morphological analysis of quinoa germplasm grown under lowland desert conditions. J Agron Crop Sci 197:124–134
González-Teuber M, Vilo C, Bascuñán-Godoy L (2017) Molecular characterization of endophytic fungi associated with the roots of Chenopodium quinoa inhabiting the Atacama Desert, Chile. Genom Data 11:109–112
Houston J, Hartley AJ (2003) The central andean west-slope rainshadow and its potential contribution to the origin of hyperaridity in the Atacama Desert. Int J Climatol 23:1453–1464
Hubbard M, Germida J, Vujanovic V (2013) Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second generation seed viability. J Appl Microbiol 116:109–122
INIA (2014) Boletín nacional de análisis de riesgos agroclimáticos para las principales especies frutales y cultivos, y la ganadería. http://newsflash.asoex.cl/userfiles/file/269
Jankovic S, Popovic V, Ikanovic J, Rakic S, Kuzevski J, Gavrilovic M (2016) Productivity traits of rye (Secale cereale), khorasan wheat (Triticum turgidum. ssp. Taranicum Mckey) and quinoa (Chenopodium quinoa Willd) grown on degraded soil. Rom Agric Res 33:283–290
Junges E, Brião Muniz MF, de Oliveira Bastos B, Oruoski P (2015) Biopriming in bean seeds. Acta Agric Scand B. https://doi.org/10.1080/09064710.2015.1087585
Kane KH (2011) Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ Exp Bot 71:337–344
Kannadan S, Rudgers JA (2008) Endophyte symbiosis benefits a rare grass under low water availability. Funct Ecol 4:706–713
Khan AL, Hamayun M, Ahmad N, Hussain J, Kang SM, Kim YH, Adnan M, Tang DS, Waqas M, Radhakrishnan R, Hwang YH, Lee IJ (2011) Salinity stress resistance offered by endophytic fungal interaction between Penicillium minioluteum LHL09 and Glycine max. L.J. Microbiol Biotechnol 21:893–902
Kleczewski NM, Bauer JT, Bever JD, Clay K, Reynolds HL (2012) A survey of endophytic fungi of switchgrass (Panicum virgatum) in the Midwest, and their putative roles in plant growth. Fungal Divers 5:521–529
Levitt J (1980) Responses of plants to environmental stress: chilling, freezing, and high temperature stresses. Academic Press, New York
López-Coria M, Hernández-Mendoza JL, Sánchez-Nieto S (2016) Trichoderma asperellum induces maize seedling growth by activating the plasma membrane H+-ATPase. Mol Plant Microbe Interact 29:797–806
Lutz M, Bascuñan-Godoy L (2017) The revival of quinoa: a crop for health. In: Waisundara V, Shiomi N (eds) Superfood and functional food—an overview of their processing and utilization. InTech, ISBN 978-953-51-5020-6, pp 37–54
Malinowski DP, Belesky DP (2000) Adaptation of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Malinowski DP, Leuchtmann A, Schmidt D, Nsberger J (1997) Growth and water status in meadow fescue (Festuca pratensis) is differently affected by its two natural endophytes. Agron J 89:673–678
Molina-Montenegro MA, Oses R, Torres-Díaz C, Atala C, Zurita-Silva A, Ruiz-Lara S (2016) Root-endophytes improve the ecophysiological performance and production of an agricultural species under drought condition. AoB PLANTS 8:plw062
Morse LJ, Day TA, Faeth SH (2002) Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of Arizona fescue under contrasting water availability regimes. Environ Exp Bot 48:257–268
Morsy MR, Oswald J, He J, Tang Y, Roossinck MJ (2010) Teasing apart a three-way symbiosis: transcriptome analyses of Curvularia protuberate in response to viral infection and heat stress. Biochem Biophys Res Commun 401:225–230
Novas VM, Collantes M, Cabrai D (2007) Environmental effects on grass-endophyte associations in the harsh conditions of south Patagonia. FEMS Microbiol Ecol 61:164–173
Noy-Meyr I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Obidiegwu JE, Glenn JB, Hamlyn GJ, Prashar A (2015) Coping with drought: stress and adaptive responses in potato and perspectives for improvement. Front Plant Sci 6:542
Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP (2014) Response of plants to water stress. Front Plant Sci 5:86
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harbor Perspect Biol 2:a001537
Radhakrishnan R, Kang S-M, Baek I-Y, Lee I-J (2014) Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J Plant Interact 9:754–762
Redman RS, Dunigan DD, Rodriguez RJ (2001) Fungal symbiosis: from mutualism to parasitism, who controls the outcome, host or invader? New Phytol 151:705–716
Redman RS, Kim YO, Woodward CJ, Greer C, Espino L, Doty SL, Rodriguez RJ (2011) Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS ONE 6:e14823
Rodriguez RJ, Redman RS (2008) More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J Exp Bot 59:1109–1114
Rodriguez RJ, Redman RS, Henson JM (2004) The role of fungal symbioses in the adaptation of plants to high stress environments. Mitig Adapt Strategies Glob Chang 9:261–272
Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y-O, Redman RS (2008) Stress tolerance in plants via habitat adapted symbiosis. ISME J 2:404–416
Rodriguez RJ, White JF Jr, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Rodriguez RJ, Woodward C, Redman RS (2010) Adaptation and survival of plants in high stress habitats via fungal endophyte conferred stress tolerance. In: Seckbach J, Grube M (eds) Symbioses and Stress. Springer, Dordrecht, pp 463–476
Rundell PW, Nobel PS (1991) Structure and function of desert root systems. In: Atkinson D (ed) Plant root growth. An ecological perspective. Blackwell, Oxford, pp 349–378
Sauer DB, Burroughs R (1986) Disinfection of seed surfaces with sodium hypochlorite. Phytopathology 76:745–749
Swarthout D, Harper E, Judd S, Bultman T (2009) Measures of leaf-level water-use efficiency in drought stressed endophyte infected and non-infected tall fescue grasses. Env Exp Bot 66:88–93
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, Franken P, Kogel KH (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102:13386–13391
Xu L, Wang AA, Wang J, Wei Q, Zhang WY (2017) Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J 5:251–258
Zarea MJ, Hajinia S, Karimi N, Mohammadi Goltapeh E, Rejali F, Varma A (2012) Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol Biochem 45:139–146
Zheng LP, Tian H, Yuan YF, Wang JW (2016) The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. J Plant Growth 80:93–102
Acknowledgements
We thank Carolina Murciano and Andrea Morales for their valuable help in the laboratory. MGT is also very grateful to Convenio de Desempeño Proyecto Basal, Universidad de Santiago de Chile. This work was supported by the Max Planck Society through the Max Planck Partner Group.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christina Birnbaum.
Rights and permissions
About this article
Cite this article
González-Teuber, M., Urzúa, A., Plaza, P. et al. Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol 219, 231–240 (2018). https://doi.org/10.1007/s11258-017-0791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-017-0791-1