Abstract
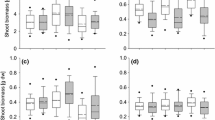
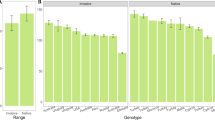
In response to novel selection pressures in an introduced range, non-native species may evolve more competitive phenotypes unique from those of their native range. We examined the existence of an invasive phenotype in the herbaceous perennial Artemisia vulgaris, a frequent invader of the Northeast and Mid-Atlantic US. Populations from both the native (European) and the introduced (North American) ranges were grown in intra-specific competition (same population), inter-specific competition with the native perennial herb Solidago canadensis, and alone in a common garden to quantify shifts in resource allocation and neighbor effects on performance and competitive ability. Without competition, introduced A. vulgaris populations were much shorter than native populations, but germinated earlier, produced more ramets, more belowground and total biomass, and maintained higher root-to-shoot ratios. Under inter- and intra-specific competitions, introduced A. vulgaris populations were shorter, but produced more ramets, belowground, and total biomass than native populations. S. canadensis belowground and total biomass were more highly suppressed by introduced than native A. vulgaris. Our data suggest that since the introduction to North America, A. vulgaris has evolved a more competitive invasive phenotype characterized by many short ramets with more extensive root/rhizome networks. This rapid evolutionary shift likely benefits A. vulgaris in its introduced range by allowing establishment and subsequent dominance in dense stands of existing vegetation.

Similar content being viewed by others
References
Barney JN (2006) North American history of two invasive plant species: phytogeographic distribution, dispersal vectors, and multiple introductions. Biol Invasions 8:703–717. doi:10.1007/s10530-005-3174-9
Barney JN, DiTommaso A (2003) The biology of Canadian weeds. 118. Artemisia vulgaris L. Can J Plant Sci 83:205–215
Barney JN, DiTommaso A, Weston LA (2005) Differences in invasibility of two contrasting habitats and invasiveness of two mugwort (Artemisia vulgaris) populations. J Appl Ecol 42:567–576. doi:10.1111/j.1365-2664.2005.01030.x
Bazzaz FA, Chiarello NR, Coley PD et al (1987) Allocating resources to reproduction and defense. BioScience 37:58–67. doi:10.2307/1310178
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042. doi:10.1890/04-0341
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889. doi:10.2307/2261425
Blumenthal DM, Hufbauer RA (2007) Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology 88:2758–2765. doi:10.1890/06-2115.1
Bossdorf O, Prati D, Auge H et al (2004) Reduced competitive ability in an invasive plant. Ecol Lett 7:346–353. doi:10.1111/j.1461-0248.2004.00583.x
Bossdorf O, Augue H, Lafuma L et al (2005) Phenotypic and genotypic differentiation between native and introduced plant populations. Oecologia 144:1–11. doi:10.1007/s00442-005-0070-z
Brown JS, Eckert CG (2005) Evolutionary increase in sexual and clonal reproductive capacity during biological invasion in an aquatic plant Butomus umbellatus (Butomaceae). Am J Bot 92:495–502. doi:10.3732/ajb.92.3.495
Caño L, Escarré J, Fleck I et al (2008) Increased fitness and plasticity of an invasive species in its introduced range: a study using Senecio pterophorus. J Ecol 96:468–476. doi:10.1111/j.1365-2745.2008.01363.x
Casper BB, Jackson RB (1997) Plant competition underground. Annu Rev Ecol Syst 28:545–570. doi:10.1146/annurev.ecolsys.28.1.545
Colautti RI, Ricciardi A, Grigorovich IA et al (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. doi:10.1111/j.1461-0248.2004.00616.x
Freckleton RP, Watkinson AR (2000) Designs for greenhouse studies of interactions between plants: an analytical perspective. J Ecol 88:386–391. doi:10.1046/j.1365-2745.2000.00467.x
Grosholz ED, Ruiz GM (2003) Biological invasions drive size increases in marine and estuarine invertebrates. Ecol Lett 6:700–705. doi:10.1046/j.1461-0248.2003.00495.x
Hairston NG, Ellner SP, Geber MA et al (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x
Hendry AP, Kinnison MT (1999) The pace of modern life: measuring rates of contemporary microevolution. Evol Int J Org Evol 53:1637–1653. doi:10.2307/2640428
Jolliffe PA (2000) The replacement series. J Ecol 88:371–385. doi:10.1046/j.1365-2745.2000.00470.x
Jolliffe PA, Minjas AN, Runeckles VC (1984) A reinterpretation of yield relationships in replacement series experiments. J Appl Ecol 21:227–243. doi:10.2307/2403049
Keddy PA, Fraser LH, Wisheu I (1998) A comparative approach to examine competitive response of 48 wetland plant species. J Veg Sci 9:777–786. doi:10.2307/3237043
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391. doi:10.1016/S0169-5347(02)02554-5
Leger EA, Rice KJ (2003) Invasive California poppies (Eschscholzia californica Cham.) grow larger than native individuals under reduced competition. Ecol Lett 6:257–264. doi:10.1046/j.1461-0248.2003.00423.x
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228. doi:10.1016/j.tree.2005.02.004
McKenney JL, Cripps MG, Price WJ et al (2007) No difference in competitive ability between invasive North American and native European Lepidium draba populations. Plant Ecol 193:293–303. doi:10.1007/s11258-007-9268-y
Mitchell CG, Power AG (2003) Release of invasive plants from fungal and viral pathogens. Nature 421:625–627. doi:10.1038/nature01317
Palumbi SR (2001) Humans as the world’s greatest evolutionary force. Science 293:1786–1790. doi:10.1126/science.293.5536.1786
Pyšek P (1997) Clonality and plant invasions: can a trait make a difference? In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants. Backhuys Publishers, Leiden, pp 405–427
Reader RJ, Wilson S, Belcher J et al (1994) Plant competition in relation to neighbor biomass: an international study with Poa pratensis. Ecology 75:1753–1760. doi:10.2307/1939634
Richards CL, Bossdorf O, Muth NZ et al (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993. doi:10.1111/j.1461-0248.2006.00950.x
Siemann E, Rogers WE (2003) Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia 135:451–457
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton
Vellend M, Harmon LJ, Lockwood JL et al (2007) Effects of exotic species on evolutionary diversification. Trends Ecol Evol 22:481–488. doi:10.1016/j.tree.2007.02.017
Vilà M, Gómez A, Maron JL (2003) Are alien plants more competitive than their native conspecifics? A test using Hypericum perforatum L. Oecologia 137:211–215. doi:10.1007/s00442-003-1342-0
Weiner J (1990) Asymmetric competition in plant-populations. Trends Ecol Evol 5:360–364. doi:10.1016/0169-5347(90)90095-U
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77:1661–1666. doi:10.2307/2265769
Wilson JB (1988) Shoot competition and root competition. J Appl Ecol 25:279–296. doi:10.2307/2403626
Acknowledgments
We would like to thank Prasanta Bhowmik, Mark Czarnota, Gilles Leroux, Art Gover, and Patricia Pingel for collecting mugwort germplasm. Comments from two anonymous reviewers improved the manuscript. J.N·B. would like to thank the Andrew W. Mellon Foundation for providing financial support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barney, J.N., Whitlow, T.H. & DiTommaso, A. Evolution of an invasive phenotype: shift to belowground dominance and enhanced competitive ability in the introduced range. Plant Ecol 202, 275–284 (2009). https://doi.org/10.1007/s11258-008-9481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9481-3