Abstract
Tumor growth is intricately linked to the process of angiogenesis, with a key role played by vascular endothelial growth factor (VEGF) and its associated signaling pathways. Notably, these pathways also play a pivotal “housekeeping” role in renal physiology. Over the past decade, the utilization of VEGF signaling inhibitors has seen a substantial rise in the treatment of diverse solid organ tumors, diabetic retinopathy, age-related macular degeneration, and various ocular diseases. However, this increased use of such agents has led to a higher frequency of encountering renal adverse effects in clinical practice. This review comprehensively addresses the incidence, pathophysiological mechanisms, and current evidence concerning renal adverse events associated with systemic and intravitreal antiangiogenic therapies targeting VEGF-A and its receptors (VEGFR) and their associated signaling pathways. Additionally, we briefly explore strategies for mitigating potential risks linked to the use of these agents and effectively managing various renal adverse events, including but not limited to hypertension, proteinuria, renal dysfunction, and electrolyte imbalances.
Similar content being viewed by others
Introduction
Pathological angiogenesis, a hallmark of tumor growth, involves the development of new blood vessels within tumors by co-opting existing ones. Numerous molecular components participate in these processes, but the central orchestrator of tumor angiogenesis is vascular endothelial growth factor (VEGF), predominantly secreted by tumor cells [1]. VEGF assumes a pivotal role as an endogenous angiogenic cytokine, serving as a central regulator of vascular growth. Its effects encompass the promotion of endothelial cell proliferation, differentiation, migration, and survival [2]. Due to its critical function, VEGF has emerged as a primary target for various therapeutic agents designed to combat cancer [3].
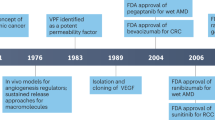
The human VEGF family comprises VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor, each displaying distinct binding affinities for VEGF receptors. Among these family members, VEGF-A, initially identified as a vascular permeability factor, governs blood vessel growth in both normal and pathological angiogenesis scenarios [4]. It communicates with endothelial cells through a VEGF receptor featuring tyrosine kinase activity, prompting them to proliferate and migrate. In the field of oncology, angiogenesis inhibitors targeting the VEGF ligand (anti-VEGF) or its receptors (tyrosine kinase inhibitors, TKIs) are widely employed. The Food and Drug Administration (FDA) approved the first antiangiogenic drug, bevacizumab (Avastin®), for clinical use in individuals with advanced colon cancer. Many of these agents have improved patient outcomes by extending overall survival and progression-free survival [1,2,3].
The VEGF system also plays a pivotal physiological role in maintaining various tissues and organs, including tissue repair, endometrial regeneration after menstruation, and inflammation [4]. Conditions such as pre-eclampsia, hemangiomas, and diabetic retinopathy are examples of vascular disorders with VEGF signaling pathways linked to their pathophysiology. The connections between anti-VEGF medications and various aspects of renal glomerular and tubular dysfunction, including proteinuria, hypertension, and electrolyte imbalances, are subjects of promising translational research [5, 6]. In this review, we aim to comprehensively examine and update the existing evidence, pathogenesis, early biomarkers of nephrotoxicity, and strategies to mitigate the renal impact of both ocular and systemic use of VEGF ligand inhibitors.
Pathophysiology of VEGF inhibition
VEGF and the eye
VEGF plays a critical role in maintaining ocular homeostasis, being produced by various cell types, including vascular endothelial cells, retinal astrocytes, retinal neurons, retinal pigmented epithelial cells, and Muller cells [7]. In pathological conditions such as hypoxia and hyperglycemia, VEGF can become overexpressed, leading to retinal angiogenesis [8]. Notably, elevated levels of vitreous and circulating VEGF have been observed in patients with both type 1 and type 2 diabetes, as well as diabetic retinopathy and diabetic nephropathy. VEGF-A has been proposed as a valuable biomarker for monitoring the progression of diabetic retinopathy [9].
The advent of VEGF inhibitors has significantly enhanced the management of various retinal ophthalmic disorders, including proliferative diabetic retinopathy, central retinal vein occlusion, diabetic macular edema, and age-related macular degeneration [10]. The intraocular administration of anti-VEGF therapy has gained widespread acceptance over the past decade [11]. Although considered a targeted therapy with minimal adverse effects and an excellent safety profile due to its specific impact on angiogenic cells with minimal harm to normal cells, it has been noted that these agents may lead to previously unknown or under-reported adverse events, particularly nephrotoxicity [12]. Anti-VEGF agents are broadly implicated in causing thrombotic microangiopathy (TMA) [13], kidney function impairment [14], or the onset or worsening of pre-existing hypertension and proteinuria [15, 16].
VEGF and the kidney
The kidneys, highly vascularized organs, are susceptible to the ischemic effects of anti-VEGF ligands. They are both a target and a source of VEGF [17]. Vascular Endothelial Growth Factor (VEGF) plays a pivotal role in maintaining the integrity of the glomerular membrane structure and facilitating communication between podocytes and endothelial cells [18]. It serves as a vital mediator in the restoration of certain renal disorders, such as non-diabetic kidney diseases, while exerting detrimental effects in others, including diabetes and its complications [19].
In the kidney, VEGF is secreted by podocytes and tubular epithelial cells and becomes biologically active upon binding to one of the VEGF receptor tyrosine kinases (RTKs), which include VEGFR-1, VEGFR-2, and VEGFR-3. VEGFR-2 primarily mediates VEGF-A signaling. These VEGF receptors are primarily expressed in podocytes and glomerular endothelial cells (ECs) [17, 20, 21]. The interaction between VEGF produced by podocytes and VEGFR-2 on glomerular ECs, referred to as paracrine epithelial-endothelial cross-talk, is vital for the normal functioning of glomeruli and the maintenance of the glomerular barrier's integrity [22, 23].
Within podocytes, autocrine VEGFA-VEGFR2 interaction stimulates nephrin-VEGFR crosstalk, regulating actin polymerization and stress fiber formation to maintain podocyte architecture. These effects are attributed to the fact that RTKs dimerize and undergo autophosphorylation in response to ligand binding, initiating downstream signaling pathways, including PI3 Kinase/AKT, Raf/MAPK/ERK, mTOR, and eNOS pathways [24,25,26]. Anti-VEGF therapy disrupts these downstream pathways, contributing to nephrotoxicity (see Fig. 1).
Pathogenesis of nephrotoxicity related to anti-VEGF therapy. Anti-VEGF drugs interfere with multiple downstream tyrosine kinase pathways responsible for maintaining glomerular and podocyte integrity. A consequence of this disruption is the development of podocyte effacement and glomerular basement membrane thickening, both leading to proteinuria. In addition, there is development of microthrombi, endotheliosis, and reduction in complement factors that lead to thrombotic microangiopathy and hypertension. Reduced fractional excretion of sodium also contributes to the hypertension. VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor, AKT-PI3 phosphoinositide-3-kinase, MAPK mitogen-activated protein kinase, ERK extracellular signal-regulated kinase, mTOR mammalian target of rapamycin, eNOS endothelial nitric oxide synthase, GBM glomerular basement membrane, MCNS minimal change nephrotic syndrome, FSGS focal segmental glomerulosclerosis, TMA thrombotic microangiopathy, CFH complement factor-H
Numerous studies have demonstrated that VEGF treatment can reduce renal illnesses and stabilize renal function in chronic kidney disease (CKD) animals [19]. Few studies showing the benefits of VEGF therapy in experimental animal models is summarized in Table 1. VEGF exhibits a nephroprotective effect in various non-diabetic renal disorders, improving renal function and reducing renal fibrosis. This suggests that inhibiting VEGF can harm podocytes and is associated with the development of glomerulosclerosis and tubulointerstitial fibrosis [27]. Therefore, VEGF may be a critical mediator in the restoration of certain renal disorders.
Anti-VEGF agents and their use in clinical practice
Understanding the VEGF-VEGFR interactions in angiogenesis has unveiled a plethora of potential therapeutic targets with applications in oncology and ophthalmology. Angiogenesis and neovascularization are critical pathological processes in these fields. VEGF and its pathways are targeted at various levels, including the reduction of VEGF gene expression to mitigate its production and secretion. Among the extensively researched areas are agents commonly referred to as anti-VEGF drugs or VEGF ligand inhibitors [32]. VEGF receptors and their inhibitors, particularly tyrosine kinase inhibitors (TKIs), also find a place in the armamentarium of oncologists [33]. For this review, we will focus on anti-VEGF/VEGF ligand inhibitor agents. Commonly used agents and their indications are summarized in Table 2.
Clinical effects of anti-VEGF drugs on kidneys and current evidence
Intravitreal anti-VEGF agents
The VEGF system plays an essential role in the pathogenesis of various ocular diseases, including proliferative retinopathies such as age-related macular degeneration, diabetic retinopathy, and central retinal vein occlusion. These diseases are characterized by ocular angiogenesis, which leads to visual impairment due to excessive VEGF activation [37, 38]. The primary goals of anti-VEGF therapy are to counteract the pathological effects of neovascularization, halt disease progression, and improve vision. Several intravitreal agents have received approval for targeting the VEGF system, including ranibizumab, aflibercept, and pegaptanib, which are commonly used. Bevacizumab, although not FDA-approved for intravitreal use, is used "off-label" for various indications by ophthalmologists. Typically, these agents are administered once monthly for 3 to 6 months, with subsequent doses adjusted based on clinical outcomes.
Pharmacokinetics of intravitreal anti-VEGF agents
When the FDA approved intravitreal aflibercept and ranibizumab for intravitreal use, it was based on studies indicating that the systemic concentrations achieved by these drugs administered intravitreally were nearly 200 times lower than required for maximal systemic VEGF inhibition [13]. Preclinical and clinical studies on the pharmacokinetics of anti-VEGF drugs have revealed that the blood-retinal barrier may contain neonatal Fc receptors (FcRn) that facilitate the transport of intravitreally administered drugs into the systemic circulation [39, 40]. This finding is supported by a rabbit model study in which intravitreal bevacizumab (1.25 mg) showed small amounts of the drug in the serum and the uninjected eye [41]. In a study involving patients with wet AMD, intravitreally administered anti-VEGF drugs exhibited systemic exposure (peak and trough concentrations, area under the curve) that was highest for bevacizumab, lowest for ranibizumab, and intermediate for aflibercept. Ranibizumab also did not show systemic accumulation despite repeated dosing, unlike the other two drugs, which showed systemic accumulation [42]. Studies on the pharmacokinetics of these drugs (bevacizumab, aflibercept, ranibizumab) have demonstrated that the peak systemic concentrations following intravitreal injection are equal to or exceed the half-maximal inhibitory concentration (IC50) levels for systemic VEGF inhibition [42, 43]. The peak concentration of intravitreal drugs may also vary depending on the underlying ophthalmic pathology, reflecting differences in retinal vascular permeability across different pathologies [42, 43].
Systemic suppression of VEGF
Evidence indicates that intravitreal anti-VEGF drugs not only achieve measurable plasma concentrations but also result in significant systemic VEGF inhibition, leading to potential systemic adverse effects. Available data suggests that aflibercept may be more potent in this regard compared to bevacizumab, with ranibizumab having the least potential for systemic VEGF inhibition [12]. Studies demonstrating systemic VEGF inhibition following intravitreal administration of these agents are summarized in Table 3. These studies reveal a notable reduction in systemic VEGF levels after intravitreal administration of the agents, with sustained suppression even one month post-injection, which may have clinical implications.
Clinical effects of intravitreal VEGF blockade on the kidneys
Despite pharmacokinetic studies suggesting sustained systemic VEGF suppression, the translation of this into clinical outcomes has yielded mixed results. Studies in monkeys have demonstrated aflibercept’s presence in the glomeruli one week after intravitreal injection [48]. Evidence for renal effects includes the development of de novo hypertension [49, 50], worsening of pre-existing hypertension [51, 52], acute kidney injury [53], and proteinuria [51, 53]. Additionally, there are over 32 biopsy-proven cases of glomerular diseases temporally associated with intravitreal anti-VEGF injections, including worsening or relapse of pre-existing glomerular pathologies and the development of de novo glomerular diseases, including collapsing FSGS and TMA [15]. Table 4 summarizes studies showing these drugs' potential renal effects. On the other hand, a few studies have shown no effect on proteinuria or GFR with the use of intravitreal anti-VEGF agents [54,55,56]. Studies by Kameda et al. and Glassman et al. included diabetic CKD patients and patients with pre-existing albuminuria, with both studies suggesting no changes in GFR, blood pressure, or albuminuria during follow-up of patients receiving anti-VEGF agents [54, 55].
Systemic agents
Most of the experience has been with bevacizumab, a humanized monoclonal antibody (IgG1) against VEGF-A, which prevents the activation of VEGFR. The VEGF family includes at least five known ligands: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor. These ligands, especially VEGF-A, bind to receptors such as VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3, along with the coreceptor neuropilins, to induce normal and tumor-associated angiogenesis [60]. In normal kidneys, VEGF is continually expressed and secreted by podocytes. Locally produced VEGF acts on VEGF receptors located on glomerular and peritubular endothelium and mesangial cells, maintaining normal glomerular function and the integrity of the glomerular basement membrane (GBM) [61]. Interfering with the paracrine function of VEGF alters local glomerular physiology, resulting in clinical and pathological manifestations of kidney damage, such as proteinuria, nephrotic syndrome, acute kidney injury, thrombotic microangiopathy (TMA), and hypertension observed with the administration of VEGF ligand inhibitors [61].
Kidney biopsies from patients treated with VEGF-ligand inhibitors have demonstrated TMA, focal segmental glomerulosclerosis, collapsing glomerulopathy (especially among patients with a history of pamidronate use), and occasional case reports of cryoglobulinemic, immune complex, and proliferative glomerulonephritis (more commonly associated with tyrosine kinase inhibitors, TKIs) [62,63,64] (Fig. 2). Complement activation can also be observed in TMA due to reduced complement factor H (CFH) and fibrin microthrombi in glomerular capillaries [65]. Although not mutually exclusive, VEGF-ligand inhibitors are associated with an increased incidence of TMA, while TKIs exhibit increased podocytopathies [66]. Furthermore, anti-VEGF therapy induces vascular resistance by reducing nitric oxide production and renal fractional sodium excretion (FENa), contributing to volume-dependent hypertension [67, 68].
A Glomerulus with features of chronic thrombotic microangiopathy. Diffuse and global duplication of glomerular basement membranes (arrows) is noted with segmental mesangial matrix expansion (PASM stain; 400×); B glomerulus showing segmental sclerosis with hyalinosis (arrow; PAS stain; 400×). Both images are representative images of lesions seen in patients with renal effects of anti-VEGF therapy and not actual patient images
The renal effects seem to be dose-dependent, with increasing nephrotoxicity observed when doses ≥ 10 mg/kg/dose (high dose) are used. In patients receiving ≤ 7.5 mg/kg/dose (low dose) bevacizumab, the incidence of proteinuria and hypertension ranged from 21 to 41% and between 2.7 and 32%, respectively. In the high dose group, an increased incidence of 22 to 63% for proteinuria and 17.6 to 36% for hypertension was observed [69]. A meta-analysis of clinical trials involving bevacizumab use in solid tumors identified an incidence of 7.9% for high-grade (grade 3 or 4) hypertension [70]. Nephrotoxicity is more frequent in patients with pre-existing renal disease, a diagnosis of renal cell carcinoma, and concurrent use of chemotherapeutic agents such as cisplatin and bisphosphonates like pamidronate [65, 69]. The package insert for bevacizumab treatment recommends temporarily withholding treatment for patients with new-onset moderate to severe proteinuria and those with uncontrolled severe hypertension. The insert also recommends permanently discontinuing treatment with bevacizumab in patients who develop nephrotic syndrome or a hypertensive crisis. Importantly, the nephrotoxic effects can be irreversible despite discontinuing the therapy [71]. A study by Li Y et al. and Roviello G et al. positively linked sorafenib-induced hypertension and ramucirumab-induced hypertension to antitumor response [72, 73]. No such predictive response was observed in the meta-analysis of seven phase 3 RCTs with bevacizumab use across multiple tumor types [74].
Predicting and mitigating renal effects of anti-VEGF drugs
No established society guidelines or studies have investigated the management of renal complications of anti-VEGF drugs or recommendations regarding a class of antihypertensives to manage proteinuria and secondary hypertension. However, prior experience from animal studies, observational studies, and case reports has shed light on potential preventive and therapeutic measures.
-
1.
Identifying patients at risk: patients with baseline comorbid conditions like hypertension, diabetes, chronic kidney disease (CKD), and proteinuria are likely to experience an exacerbation of underlying kidney disease when using these agents [16, 50]. Hence, these patients may be considered “at risk” and should be planned for close monitoring.
-
2.
Monitoring for renal side effects during therapy: once at-risk patients are identified, regular monitoring is crucial after receiving anti-VEGF therapy. Active blood pressure monitoring and albuminuria measurement, before and after initiation of therapy, are of prime importance [75]. Any worsening of hypertension, proteinuria, or creatinine levels may indicate a renal effect, and the patient should undergo further assessment, including a renal biopsy. A detailed discussion should ensue with the patient, explaining the risks and benefits of continuing VEGF-ligand inhibitors in the presence of ongoing renal injury. Discontinuation of bevacizumab can reduce proteinuria and improve hypertension control. The use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, as in the general population, to reduce proteinuria and lower intra-glomerular pressure, may not replicate similarly in patients on anti-VEGF therapy. Siddique et al. demonstrated inhibition of VEGF ligand and VEGFR expression in the myocardium of rats exposed to enalapril or candesartan [76].
-
3.
Biomarkers of nephrotoxicity: chebotareva and colleagues examined various urinary biomarkers and their ability to predict nephrotoxicity in patients exposed to anti-VEGF ligands. Elevated urinary biomarkers at the end of the first and second week following ranibizumab/bevacizumab/aflibercept administration predicted the clinical occurrence of nephrotoxicity at week 8 with over 65% sensitivity and specificity. Urinary Neutrophilic Gelatinase-Associated Lipocalin (NGAL), expressed significantly from the distal segment of the nephron; urinary Kidney Injury Molecule 1 (KIM-1), a proximal tubular transmembrane protein; Hypoxia Inducible Factor-1α (HIF-1α), reflecting rarefaction of peritubular capillaries and renal tissue hypoxia; and Nephrin, indicating a break in the glomerular filtration barrier, were elevated following a single dose of anti-VEGF ligand, suggesting injury across different nephron segments [77].
-
4.
Treatment of renal side effects: in patients identified as potentially having developed anti-VEGF therapy-induced renal side effects such as proteinuria, hypertension, or worsening creatinine, no proven therapies can directly facilitate these changes. Managing hypertension and proteinuria with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEi/ARBs) may be a prudent approach. However, reducing the dose of the anti-VEGF agent, using alternate agents, or stopping drug therapy may be necessary to prevent further damage [12]. As stated previously, nephrotoxicity of bevacizumab is dose-dependent and is more often observed when the dose is ≥ 10 mg/kg/dose, and nephrotoxicity may be irreversible despite stopping the drug.
Conclusion and future directions
Anti-angiogenic therapy stands as a promising and innovative approach for addressing conditions reliant on angiogenesis. Specifically, inhibitors targeting vascular endothelial growth factor (VEGF) have emerged as powerful tools in managing angiogenesis-dependent disorders like cancer and diabetic retinopathy, due to their capacity to inhibit angiogenesis. As VEGF plays a crucial role in maintaining renal homeostasis, the use of VEGF ligand inhibitors has been associated with various renovascular conditions. These conditions manifest as proteinuria, hypertension, nephrotic syndrome, reduced glomerular filtration rate (GFR), and thrombotic microangiopathy (TMA). The identification of individuals at risk for nephrotoxicity, the utilization of urinary biomarkers as indicators of renal injury, and the implementation of strategies to minimize exposure to high-dose VEGF ligand inhibitors collectively contribute to improving renal outcomes.
References
Zhang W, Feng L-J, Teng F, Li Y-H, Zhang X, Ran Y-G (2020) Incidence and risk of proteinuria associated with newly approved vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: an up-to-date meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol 13:311–320
Semeniuk-Wojtaś A, Lubas A, Stec R, Szczylik C, Niemczyk S (2016) Influence of tyrosine kinase inhibitors on hypertension and nephrotoxicity in metastatic renal cell cancer patients. Int J Mol Sci 17:2073
Abbas A, Mirza MM, Ganti AK, Tendulkar K (2015) Renal toxicities of targeted therapies. Target Oncol 10:487–499
Shibuya M (2014) VEGF-VEGFR signals in health and disease. Biomol Ther (Seoul) 22:1–9
Izzedine H, Mangier M, Ory V, Zhang S-Y, Sendeyo K, Bouachi K et al (2014) Expression patterns of RelA and c-mip are associated with different glomerular diseases following anti-VEGF therapy. Kidney Int 85:457–470
Calizo RC, Bhattacharya S, van Hasselt JGC, Wei C, Wong JS, Wiener RJ et al (2019) Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat Commun 10:2061
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27:331–371
Al-Kharashi AS (2018) Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol 32:318–323
Ahuja S, Saxena S, Akduman L, Meyer CH, Kruzliak P, Khanna VK (2019) Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int J Retina Vitreous 5:29
Stewart MW (2012) The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc 87:77–88
Cornel S, Adriana ID, Mihaela TC, Speranta S, Algerino DS, Mehdi B et al (2015) Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol 59:235–242
Hanna RM, Barsoum M, Arman F, Selamet U, Hasnain H, Kurtz I (2019) Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int 96:572–580
Phadke G, Hanna RM, Ferrey A, Torres EA, Singla A, Kaushal A et al (2021) Review of intravitreal VEGF inhibitor toxicity and report of collapsing FSGS with TMA in a patient with age-related macular degeneration. Clin Kidney J 14:2158–2165
Shye M, Hanna RM, Patel SS, Tram-Tran N, Hou J, Mccannel C et al (2020) Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin Kidney J 13:969–980
Hanna RM, Ahdoot RS, Kim MS, Jhaveri KD, Kalantar-Zadeh K, Kurtz IB (2022) Intravitreal vascular endothelial growth factors hypertension, proteinuria, and renal injury: a concise review. Curr Opin Nephrol Hypertens 31:47–56
Hanna RM, Lopez EA, Hasnain H, Selamet U, Wilson J, Youssef PN et al (2019) Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J 12:92–100
Schrijvers BF, Flyvbjerg A, De Vriese AS (2004) The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65:2003–2017
Ollero M, Sahali D (2015) Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol Dial Transplant 30:1449–1455
Guise E, Chade AR (2018) VEGF therapy for the kidney: emerging strategies. Am J Physiol-Renal Physiol 315:F747–F751
Eremina V, Baelde HJ, Quaggin SE (2007) Role of the VEGF–a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol 106:p32–p37
Müller-Deile J, Worthmann K, Saleem M, Tossidou I, Haller H, Schiffer M (2009) The balance of autocrine VEGF-A and VEGF-C determines podocyte survival. Am J Physiol Renal Physiol 297:F1656–F1667
Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE (2012) Vegfa protects the glomerular microvasculature in diabetes. Diabetes 61:2958–2966
Tanabe K, Wada J, Sato Y (2020) Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat Rev Nephrol 16:289–303
Leonard EC, Friedrich JL, Basile DP (2008) VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295:F1648–F1657
Kang D-H, Hughes J, Mazzali M, Schreiner GF, Johnson RJ (2001) Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12:1448–1457
Chade AR (2016) Vascular endothelial growth factor therapy for the kidney: are we there yet? J Am Soc Nephrol 27:1–3
Logue OC, McGowan JWD, George EM, Bidwell GL (2016) Therapeutic angiogenesis by vascular endothelial growth factor supplementation for treatment of renal disease. Curr Opin Nephrol Hypertens 25:404–409
Suga S-I, Kim Y-G, Joly A, Puchacz E, Kang D-H, Jefferson JA et al (2001) Vascular endothelial growth factor (VEGF121) protects rats from renal infarction in thrombotic microangiopathy. Kidney Int 60:1297–1308
Kang D-H, Hughes J, Mazzali M, Schreiner GF, Johnson RJ (2001) Impaired angiogenesis in the remnant kidney model. J Am Soc Nephrol 12:1448–1457
Kang D-H, Kim Y-G, Andoh TF, Gordon KL, Suga S-I, Mazzali M et al (2001) Post-cyclosporine-mediated hypertension and nephropathy: amelioration by vascular endothelial growth factor. Am J Physiol-Renal Physiol 280:F727–F736
Chade AR, Kelsen S (2012) Reversal of renal dysfunction by targeted administration of VEGF into thestenotic kidney: a novel potential therapeutic approach. Am J Physiol-Renal physiol 302(10):F1342–F1350. https://doi.org/10.1152/ajprenal.00674.2011
Ferrara N, Mass RD, Campa C, Kim R (2007) Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med 58:491–504
Cardones AR, Banez LL (2006) VEGF inhibitors in cancer therapy. Curr Pharm Des 12:387–394
Ellis LM (2006) Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol 33:S1-7
Heier JS, Brown DM, Chong V, Korobelnik J-F, Kaiser PK, Nguyen QD et al (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R (2018) Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond) 32:1010–1020
Das A, McGuire PG (2003) Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res 22:721–748
Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN (2004) An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol 138:S19-31
Kim H, Robinson SB, Csaky KG (2009) FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis 15:2803–2812
Powner MB, McKenzie JAG, Christianson GJ, Roopenian DC, Fruttiger M (2014) Expression of neonatal Fc receptor in the eye [Internet]. Investigative ophthalmology; visual science. UCL Institute of Ophthalmology, University College London, London, United Kingdom. p. 1607–15. Available from: http://europepmc.org/abstract/MED/24550358 Accessed 24 Nov 2023.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ (2007) Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 114:2179–2182
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98:1636–1641
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R et al (2017) Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab and ranibizumab. Retina 37:1847–1858
Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R et al (2015) Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 93:e154–e159
Yoon MH, Kim YJ, Lee SY, Lee KW, Chin HS (2016) Effects of intravitreal injection of bevacizumab or ranibizumab on systemic circulation. jkos 57:429–437. https://doi.org/10.3341/jkos.2016.57.3.429
Rogers CA, Scott LJ, Reeves BC, Downes S, Lotery AJ, Dick AD et al (2018) Serum vascular endothelial growth factor levels in the IVAN trial; relationships with drug, dosing, and systemic serious adverse events. Ophthalmol Retina 2:118–127
Hirano T, Toriyama Y, Iesato Y, Imai A, Murata T (2018) Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 38:1801–1808
Tschulakow A, Christner S, Julien S, Ludinsky M, van der Giet M, Schraermeyer U (2014) Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS ONE 9:e113701. https://doi.org/10.1371/journal.pone.0113701
Lee K, Yang H, Lim H, Lew HM (2009) A prospective study of blood pressure and intraocular pressure changes in hypertensive and nonhypertensive patients after intravitreal bevacizumab injection. Retina 29:1409–1417
Rasier R, Artunay O, Yuzbasioglu E, Sengul A, Bahcecioglu H (2009) The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 23:1714–1718
Bagheri S, Dormanesh B, Afarid M, Sagheb MM (2018) Proteinuria and renal dysfunction after intravitreal injection of bevacizumab in patients with diabetic nephropathy: a prospective observational study. Galen Med J 7:e1299
Shah AR, Van Horn AN, Verchinina L, Wichorek M, Su L, Markel D et al (2019) Blood pressure is associated with receiving intravitreal anti-vascular endothelial growth factor treatment in patients with diabetes. Ophthalmol Retina 3:410–416
Jalalonmuhali M, TengkuKamalden TAF, Ismail Ain S, Yong SY, Teo WT, Lim SK (2020) P0590ADVERSE renal outcome following administration of intravitreal anti-vascular endothelial growth factor inhibitors in a single tertiary centre in Malaysia. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa142.P0590
Glassman AR, Liu D, Jampol LM, Sun JK (2018) Changes in blood pressure and urine albumin-creatinine ratio in a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. Invest Ophthalmol Vis Sci 59:1199–1205
Kameda Y, Babazono T, Uchigata Y, Kitano S (2018) Renal function after intravitreal administration of vascular endothelial growth factor inhibitors in patients with diabetes and chronic kidney disease. J Diabetes Investig 9:937–939
O’Neill RA, Gallagher P, Douglas T, Little J-A, Maxwell AP, Silvestri G et al (2019) Evaluation of long-term intravitreal anti-vascular endothelial growth factor injections on renal function in patients with and without diabetic kidney disease. BMC Nephrol 20:478. https://doi.org/10.1186/s12882-019-1650-1
Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ et al (2007) A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 114:1860–1867
Hanna RM, Abdelnour L, Hasnain H, Selamet U, Kurtz I (2020) Intravitreal bevacizumab-induced exacerbation of proteinuria in diabetic nephropathy, and amelioration by switching to ranibizumab. SAGE Open Med Case Rep 8:2050313X20907033
Hanna RM, Tran N-T, Patel SS, Hou J, Jhaveri KD, Parikh R et al (2020) Thrombotic microangiopathy and acute kidney injury induced after intravitreal injection of vascular endothelial growth factor inhibitors VEGF blockade-related TMA after intravitreal use. Front Med (Lausanne) 7:579603
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611
Launay-Vacher V, Deray G (2009) Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs 20:81–82
George BA, Zhou XJ, Toto R (2007) Nephrotic syndrome after bevacizumab: case report and literature review. Am J Kidney Dis 49:e23–e29
Usui J, Glezerman IG, Salvatore SP, Chandran CB, Flombaum CD, Seshan SV (2014) Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: a report of 5 cases and review of literature. Hum Pathol 45:1918–1927
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J et al (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358:1129–1136
Zhao N, Xu Q, Wang M, Fei X, Pan Y, Chen X et al (2014) Mechanism of kidney injury caused by bevacizumab in rats. Int J Clin Exp Pathol 7:8675–8683
Estrada CC, Maldonado A, Mallipattu SK (2019) Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol 30:187–200
Grisk O, Koenen A, Meissner T, Donner A, Braun D, Steinbach A et al (2014) Rho kinase inhibition mitigates sunitinib-induced rise in arterial pressure and renal vascular resistance but not increased renal sodium reabsorption. J Hypertens 32:2199–2210
Witte J, Lampe J, Koenen A, Urbaneck I, Steinbach A, Rettig R et al (2018) The role of distal tubule and collecting duct sodium reabsorption in sunitinib-induced hypertension. J Hypertens 36:892–903
Zhu X, Wu S, Dahut WL, Parikh CR (2007) Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49:186–193
Ranpura V, Pulipati B, Chu D, Zhu X, Wu S (2010) Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 23:460–468
Launay-Vacher V, Aapro M, De Castro G, Cohen E, Deray G, Dooley M et al (2015) Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN). Ann Oncol 26:1677–1684
Roviello G, Corona SP, Multari AG, Paganini G, Chiriacò G, Conca R et al (2018) Association between ramucirumab-related hypertension and response to treatment in patients with metastatic gastric cancer. Oncotarget 9:22332–22339
Li Y, Li S, Zhu Y, Liang X, Meng H, Chen J et al (2014) Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 16:177–185
Hurwitz HI, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA et al (2013) Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist 18:273–280
Maitland ML, Bakris GL, Black HR, Chen HX, Durand J-B, Elliott WJ et al (2010) Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 102:596–604
Siddiqui AJ, Mansson-Broberg A, Gustafsson T, Grinnemo KH, Dellgren G, Hao X et al (2005) Antagonism of the renin-angiotensin system can counteract cardiac angiogenic vascular endothelial growth factor gene therapy and myocardial angiogenesis in the normal heart. Am J Hypertens 18:1347–1352
Chebotareva N, Grechukhina K, Mcdonnell V, Zhukova L, Krasnova T (2022) Early biomarkers of nephrotoxicity associated with the use of anti-VEGF drugs. Biomed Rep 16:46
Acknowledgements
We thank Dr Kiran K, Consultant Nephropathologist, Aster Whitefield Hospitals, Bengaluru for provided us the histopathology images.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests. No financial help was taken for preparation of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rangaswamy, D., Nagaraju, S.P., Bhojaraja, M.V. et al. Ocular and systemic vascular endothelial growth factor ligand inhibitor use and nephrotoxicity: an update. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-03990-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-03990-1